Summary of Research Program
A fundamental challenge for both basic and clinical neuroscience is to address how specific neuron types and neural pathways relate to distinct behavioral and physiological responses, therefore, define their function. The main goal of our research program is to address aspects of this question by elucidating the function of specific hippocampal pathways (Part 1) and GABA interneurons subtypes (Part 2) in sensory, emotional and cognitive control in behavioral contexts. Under this overarching theme, we discovered that there are separable circuits and interneuron populations embedded in the hippocampus and prefrontal cortex, working together to guide anxiety, stress, olfaction, and memory, often with opposing functions. In parallel, we have also contributed to the development of new recombinase-based genetic tools that have offered far-reaching capabilities for noninvasively labeling and silencing neuron subtypes of choice.
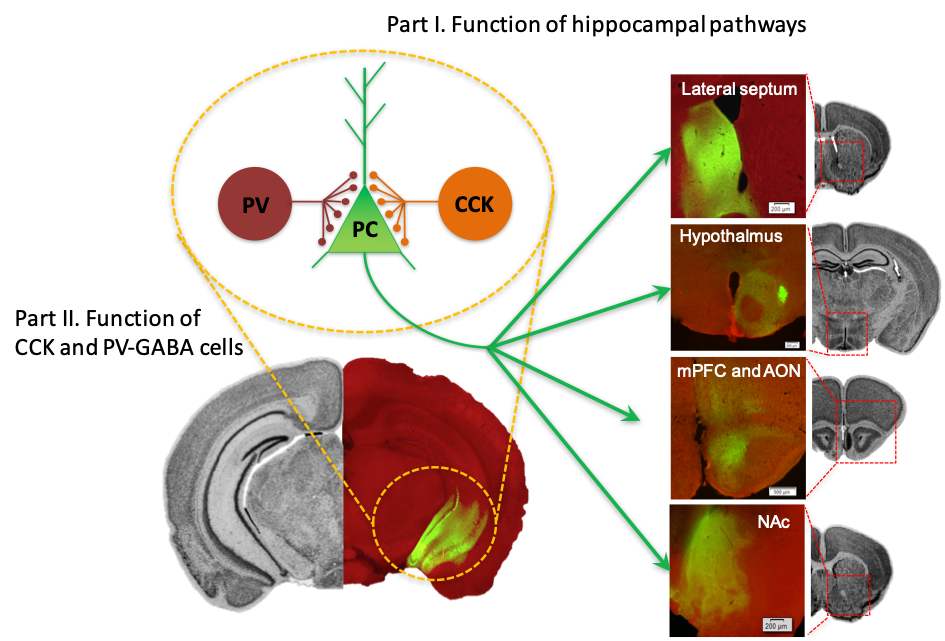
Neural pathways arising from the ventral hippocampus (vHPC). Left, virally-mediated expression of channel rhodopsin-GFP in pyramidal cells (PC) in the vHPC. PV- and CCK-GABA interneurons form synapses on the PC around the soma and proximal dendrites. Right, vHPC-originated axon terminals at its remote targets including the lateral septum, hypothalamus, medial prefrontal cortex (mPFC), anterior olfactory nucleus (AON), and nucleus accumbens (NAc).
The coordinated firing pattern of principal cells, as a neural representation of current and past experiences, is sculpted extensively by local inhibitory interneurons in the brain. The processed neural information then propagates to remote target areas along distinct axon projection pathways to produce diverse behavioural and physiological responses. In part I of our research program, we investigate the role of neural information arising from different hippocampal pathways in behavioural contexts. In part II, we study how hippocampus-dependent behaviours are modulated by the activity of local inhibitory interneurons, focusing on two genetically distinct subtypes of basket cells, namely PV- and CCK-GABA cells.
Part I. Role of hippocampal pathways in shaping anxiety, stress, and olfactory processing.
Much of the study of the mammalian hippocampus has been focused on its role in cognitive functions such as episodic memory and spatial mapping. Emerging research, however, shows that hippocampus is also a major component of circuits underlying emotional control. The dominant view is that these two (cognitive vs. emotional) functions map onto different subregions of hippocampus, where the dorsal part (dHPC) mediates cognitive functions while its ventral portion (vHPC) plays a more influential role in emotional responses. Consistent with the proposed functional heterogeneity within the HPC, the connectivity of the HPC also changes significantly along its dorsoventral axis. The dHPC has strong direct connections to the perirhinal and entorhinal cortex that carry the major inputs of visual and spatial information. On the other hand, the vHPC projects to the prefrontal cortex, hypothalamus, amygdala, nucleus accumbens, as well as other subcortical structures that are associated with fear, anxiety, stress response, and motivation.
Although the accumulated evidence unequivocally shows that the vHPC plays a critical role in controlling emotion, a circuit-level understanding of how it engages in relevant behavioural and physiological responses is lacking. Our working model is that depending on the locus of hippocampal outputs, specific hippocampal pathways subserving different functions are recruited, and each pathway activates a unique set of downstream targets, producing distinct behavioral and physiological outcomes. In this regard, elucidating the function of specific hippocampal pathways will provide an essential entry point for understanding how the hippocampus supports a diverse range of emotional behaviours. Thus, a main objective of my research program (Part 1) is to determine the behavioural function of hippocampal pathways and identify their downstream targets underlying emotional control, with a particular focus on anxiety, stress responses, and olfactory processing.
Part II. Behavioural function of local GABA interneurons
We have shown that HPC outputs to its different remote targets play specialized roles in modulating diverse behavioral processes. Importantly, however, the output signals of the HPC pathways are processed extensively by local GABAergic transmission before they propagate to remote targets. Thus, elucidating the precise function performed by local GABAergic transmission is a critical step in understanding the HPC control of behaviours. The local GABAergic transmission is orchestrated by a range of interneuron subtypes that possess different morphological, electrophysiological and neurochemical properties, and specific connectivity features. Indeed, recent years have witnessed a dramatic accumulation of our knowledge about a division of labor among distinct subtypes of interneuron where distinct interneuron subtypes specialize in inhibiting particular classes of neurons and synapsing on different subcellular compartments at precise time windows.
By far, the most extensively studied interneurons in this regard are the basket cells which synapse on pyramidal cell bodies or proximal dendrites. These basket cells are either parvalbumin (PV)- or cholecystokinin (CCK)-positive, i.e. PV- or CCK-GABA cells. The non-overlapping expression pattern of PV and CCK in basket cells is accompanied by their contrasting firing patterns and synaptic features. PV-GABA cells have fast, non-adaptive firing patterns and form synapses on pyramidal cells predominantly via α1 subunit-containing GABAa receptor with fast IPSC decay kinetics, which makes PV-GABA cells ideal for operating as a non-plastic clockwork in shaping and coordinating fast network oscillation such as gamma rhythm. In contrast, CCK-GABA cells display a slower, more accommodating firing pattern and inhibit pyramidal cells predominantly via α2 subunit-containing GABAa receptor with relatively slow and asynchronous IPSC, which makes them more amenable to synaptic modulation and plasticity and suitable for coordinating slower, theta frequency oscillation.
These contrasting features have stirred speculation that CCK- and PV-GABA neurons serve distinct roles, not only during information processing at a local circuit level but also in shaping task-dependent activity even at behavioural level. Our research program has been tackling this question, using chemo- and optogenetic methods combined with novel recombinase-based gene delivery. Of note, PV-GABA cells have been extensively studied by many labs over the last decade, showing their role in a wide variety of cognitive processes, including gamma rhythm generation, attention, novelty recognition, associative learning and extinction of learned behavior, and working memory, to name a few. In contrast, CCK-GABA cells have been less scrutinized, and their precise role in emotional and cognitive processes remains largely unknown. Addressing this knowledge gap has been a major goal of our research program.