Quiz 1C KEY - Faculty.piercecollege.edu
Quiz 1C KEY - Faculty.piercecollege.edu
Quiz 1C KEY - Faculty.piercecollege.edu
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
<strong>KEY</strong><br />
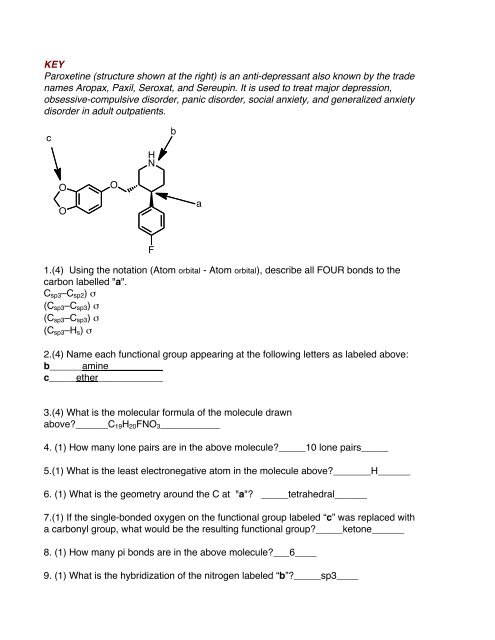
Paroxetine (structure shown at the right) is an anti-depressant also known by the trade<br />
names Aropax, Paxil, Seroxat, and Sereupin. It is used to treat major depression,<br />
obsessive-compulsive disorder, panic disorder, social anxiety, and generalized anxiety<br />
disorder in adult outpatients.<br />
c<br />
O<br />
O<br />
O<br />
H<br />
N<br />
F<br />
1.(4) Using the notation (Atom orbital - Atom orbital), describe all FOUR bonds to the<br />
carbon labelled "a".<br />
Csp3–Csp2) σ<br />
(Csp3–Csp3) σ<br />
(Csp3–Csp3) σ<br />
(Csp3–Hs) σ<br />
2.(4) Name each functional group appearing at the following letters as labeled above:<br />
b______amine__________<br />
c_____ether____________<br />
3.(4) What is the molecular formula of the molecule drawn<br />
above?______C19H20FNO3___________<br />
b<br />
4. (1) How many lone pairs are in the above molecule?_____10 lone pairs_____<br />
5.(1) What is the least electronegative atom in the molecule above?_______H______<br />
6. (1) What is the geometry around the C at "a"? _____tetrahedral______<br />
7.(1) If the single-bonded oxygen on the functional group labeled “c” was replaced with<br />
a carbonyl group, what would be the resulting functional group?_____ketone______<br />
8. (1) How many pi bonds are in the above molecule?___6____<br />
a<br />
9. (1) What is the hybridization of the nitrogen labeled “b”?_____sp3____
10. (10) Draw two additional resonance structures for molecule “A” in the boxes<br />
provided.<br />
OH OH OH<br />
A<br />
11. (1) Of the two contributors that you drew in question 10, which is more significant?<br />
Circle a letter: B or C ?<br />
12. (3) In the space below, draw the resonance hybrid of A, B, and C.<br />
δ+ δ+<br />
OH<br />
δ+<br />
B C<br />
13. (4) In the space below, draw the VSEPR diagram for tetrafluoromethane, CF4.<br />
Indicate which bonds are polar by drawing dipole arrows parallel to the bond. In the<br />
space next to the molecule, draw the direction of the net dipole (if any) or write “no net<br />
dipole”.<br />
F<br />
F<br />
F F<br />
all 4 dipoles cancel out due to symmetry - no net dipole<br />
14. (4) By substituting, adding, subtracting, or otherwise changing no more than ONE<br />
atom of tetrafluoromethane, draw a molecule with a HIGHER boiling point than<br />
tetrafluoromethane..<br />
2
F<br />
F<br />
H F<br />
No longer symmetric - net dipole results in higher BP<br />
3