New evidence for coiling direction of benthic foraminifera as a temperature proxy
- 1Laboratory of Marine Organism Taxonomy and Phylogeny, Institute of Oceanology, Chinese Academy of Sciences; Center for Ocean Mega-Science, Chinese Academy of Sciences, Qingdao, China
- 2Southern Marine Science and Engineering Guangdong Laboratory (Zhuhai), Zhuhai, China
- 3University of Chinese Academy of Sciences, Beijing, China
Foraminifera are sensitive to climate change and their species composition, shell chemical element composition and morphological characteristics are useful paleoenvironmental proxies. Coiling direction is a distinctive and easily identifiable morphological feature in trochospiral foraminifera and has been used for paleoceanographic reconstruction. Here, we conducted a field survey in a low intertidal zone in Yellow Sea for 13 months and performed a culture experiment under three temperatures and four salinities for the benthic foraminifera to seek the relationship between coiling direction and environmental factors. Our results showed that the dominant benthic foraminifera Ammonia aomoriensis (Asano, 1951) preferred sinistral direction under high temperature and had no preference with salinity. Statistical analysis showed that the ratio of sinistral/dextral in A. aomoriensis was significantly positively correlated with temperature (r = 0.5017, p = 0.0011 for field survey and r = 0.5117, p = 0.0014 for culture experiment), but had no evident relationship with salinity (p > 0.05). The ratio of sinistral/dextral was significantly negatively related with the abundance of A. aomoriensis (p < 0.05) and the ratio of sinistral/dextral was significantly positively related with the size (p < 0.05). This was the first study on the coiling direction of benthic foraminifera combining the field survey and culture experiment. Our findings suggested that the ratio of sinistral/dextral in A. aomoriensis could be used to indicate the change of temperature. This study offered new evidence for the reliability of the coiling direction as a temperature proxy and made us rethink the significance of the morphological change in biological adaptation and evolution.
1 Introduction
Foraminifera are a group of single-cell protists and distribute in nearly all marine environments. Most foraminifera build calcareous shells with calcium carbonate and these shells can be preserved in the sediments for a long time. Foraminifera are also sensitive to environmental changes (e.g., Boehnert et al., 2020). Their species composition, chemical element composition and morphologic changes has been widely used in environmental and paleoceanographic reconstruction (e.g., Saraswat, 2015; Romano et al., 2021). The morphology method is non-destructive compared to the chemical analysis of the shells. The size, deformity and coiling direction were the most commonly used proxies in foraminifera.
The growth of foraminifera is accompanied by the addition of chambers. In some foraminiferal taxa, their chambers are arranged in trochospiral manner and then two coiling directions (sinistral and dextral, i.e. anticlockwise and clockwise in the dorsal view, respectively) exist (Saraswat, 2015; Lei et al., 2017). Coiling direction is a very distinct characteristic and it can be easily recognized without specialized knowledge of taxonomy and morphology. The study of coiling direction in foraminifera had a long history and early work mainly focused on planktic foraminifera in sediments (Ericson, 1959). Saito (1976) found the coiling direction of foraminifera varied with the stratigraphic position and other researchers established the relationships between coiling direction and temperature (Jenkins, 1967; Herman, 1972; Desmares et al., 2016).
The coiling direcitons of planktic foraminifera genera Globorotalia and Neogloboquadrina are the most studied. Previous study showed in Globorotalia truncatulinoides, sinistral populations predominated when warm and dextral forms more when cold (Herman, 1972). And the ratio of sinistral/dextral of G. truncatulinoides was successfully used as an indicator for ocean circulation (Billups et al., 2016). However, another study showed that coiling direction was not controlled by environment (Norris and Nishi, 2001). For example, the coiling direction of planktic foraminifera G. truncatulinoides and N. pachyderma was determined by gene rather than temperature (Darling et al., 2006; Ujiié et al., 2010; Ujiié and Asami, 2014). Other studies also reported that the coiling direction was related to the reproduction of foraminifera, but different and even opposite results were observed in different studies (Linshy et al., 2007; Khare et al., 2012; Davis et al., 2020). Therefore, there is no uniform and convincing conclusion about what factors control coiling direction and previous studies cannot validate or disprove the opinions of environmental or genetic control (Luciani et al., 2021). Among these previous studies, most focused on planktic foraminifera and little attention had been paid to benthic foraminifera, especially the living individuals (Hallock and Rosenkrands Larsen, 1979; Nigam and Khare, 1992; Galeotti and Coccioni, 2002).
Ammonia aomoriensis (Asano, 1951) is a cosmopolitan species of benthic foraminifera and also a dominant species in shallow water, especially the intertidal zone (Schönfeld et al., 2021). Previous studies showed that the abundance, size and deformity ratio of A. aomoriensis could indicate environmental changes, including temperature, salinity, pH, and so on (Dong et al., 2019; Dong et al., 2020; Li et al., 2020). To date, no relationship between the coiling ratio of A. aomoriensis and environmental factors was established (Lei et al., 2017).
In this study, we investigated the living A. aomoriensis in an intertidal zone of Yellow Sea for 13 months and designed a culture experiment to verify the relationships between the direction ratio of A. aomoriensis and temperature and salinity. This work aimed to study the significance and reliability of the coiling ratio for benthic foraminifera as an environmental indicator. Our present data provided new evidence and relationships for the coiling direction of benthic foraminifera as a temperature proxy and the usefulness in paleoceanographic reconstruction.
2 Materials and methods
2.1 Study area and sampling
The study area was an intertidal zone in Yellow Sea (36.06°N, 120.32°E). It was a sandy beach in Qingdao and was slightly affected by tourism activities in summer. No specific permits or approvals were required to collect sediments in this area. The sample site was not privately owned or protected in any way. No endangered or protected species were involved. We collected the surface sediment (0–1 cm) samples using a sampling spoon for continued 13 months from January 2017 to January 2018 during the ebb tide time. Three repetitions (~10 g) were collected for each sample and there was a total of 39 samples for field survey study. The sediment samples were stored in plastic bottles and fixed using 95% ethanol mixed with 1g L−1 Rose Bengal to distinguish the live and dead foraminiferal specimens. During the sampling, the temperature and salinity of in situ seawater were measured.
2.2 Culture experiment
Undisturbed surface sediments containing the whole benthic foraminiferal community in the study area were collected in July and transported to laboratory. In laboratory, the sediment samples were evenly mixed and randomly divided into 36 aliquots of 10 g wet weight using sampling spoon. We designed a culture experiment with three temperatures (6, 12 and 18°C) and four salinities (15, 20, 25 and 30 psu). There were three repetitions under each treatment, yielding 36 samples corresponding to the previous 36 aliquots. The culture experiment was performed in incubators with a constant temperature. The desired salinities were adjusted with deionized water. The seawater was changed twice weekly to maintain a constant salinity and frozen concentrated Nitzschia closterium (collected from the study area) was added simultaneously as the food for benthic foraminifera. The experimental benthic foraminifera were acclimated to different temperature and salinity conditions for a month, then the formal experiment began. The formal culture experiment time was 10 weeks. After experiment, all cultured sediments were fixed with 95% ethanol mixed with 1g L−1 Rose Bengal as previously described.
2.3 Sample treatment
All sediment samples were dried in the oven at 50°C and weighted and sieved with 150 μm meshes. Living A. aomoriensis specimens (Rose-Bengal stained) > 150 μm were picked out, counted by the coiling direction (Figure 1). Smaller individuals (< 150 μm) were preserved for future studies. The maximum diameter of each specimen (i.e., shell size) were measured under a stereomicroscope (Olympus SZX16 with cellSens Standard software).
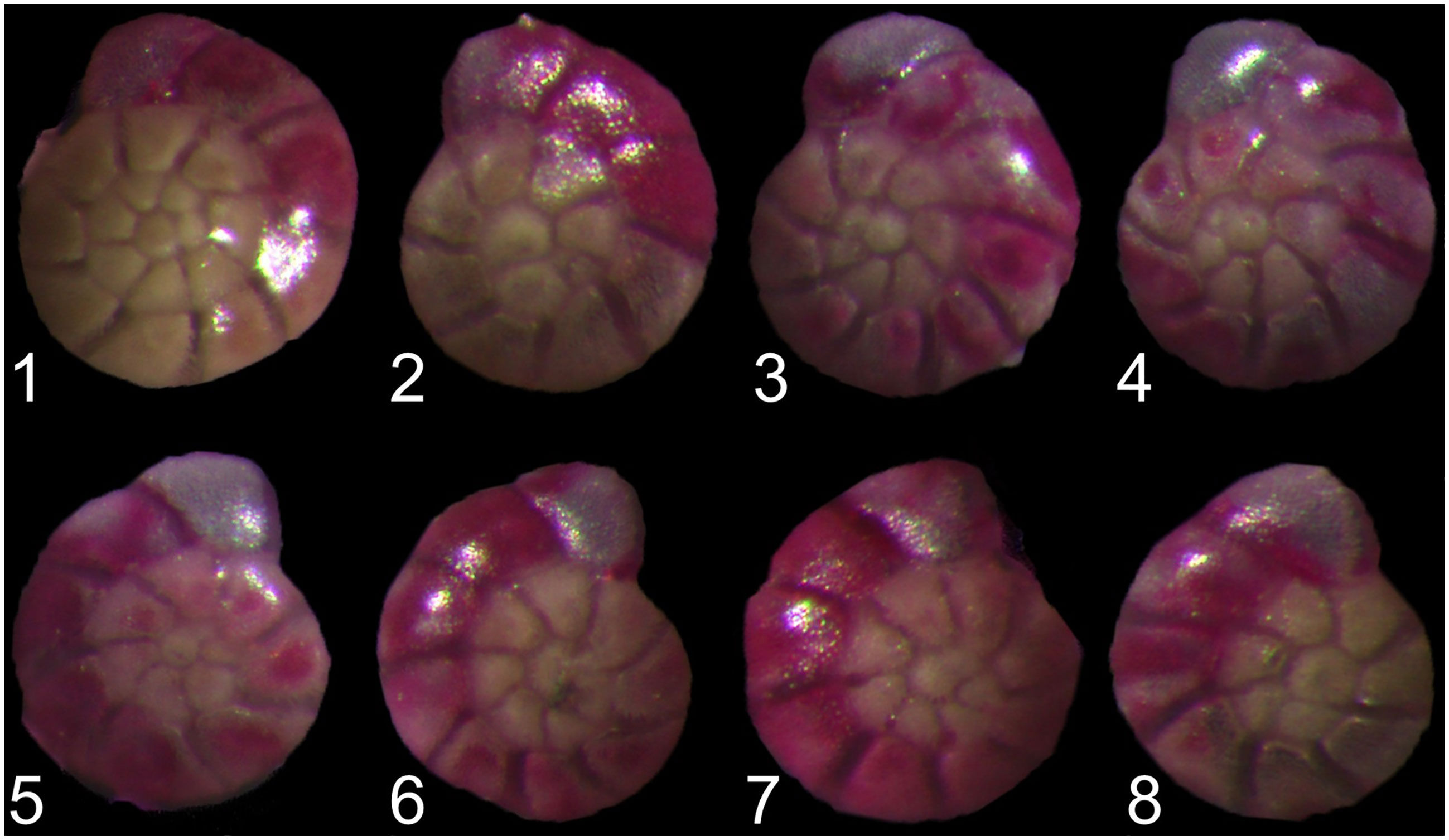
Figure 1 Ammonia aomoriensis (Asano, 1951) speciemens with sinistral (1–4) and dextral (5–8) coiling direction.
2.4 Statistical analysis
The abundance of A. aomoriensis was calculated as the number of individuals in 1 g dry weight of sediment (ind./g DW) for the field survey and that in 1 g wet weight of sediment (ind./g WW) for the culture experiment, respectively. We used a two-way ANOVA to test the effects of temperature or salinity on coiling direction and used Tukey’s Studentized Range (HSD) as a post hoc test method. Pearson correlation was analyzed between environmental factor and coiling direction. These analyses were performed using OriginPro 2021 and SAS 9.2 for Windows version.
3 Results
3.1 Field survey
The seawater temperature varied greatly monthly and ranged from 3.6 to 25.8°C, averaging 15.5°C in the study area (Figure 2A). The salinity varied from 29.5 to 33.5 psu, averaging 31.4 psu (Figure 2B). A total of 2652 live A. aomoriensis were obtained in 13 months’ field survey. Among them, 1369 were sinistral and 1283 were dextral. The ratios of sinistral/dextral were between 0.92 and 1.54 over 13 months (Figure 2C).
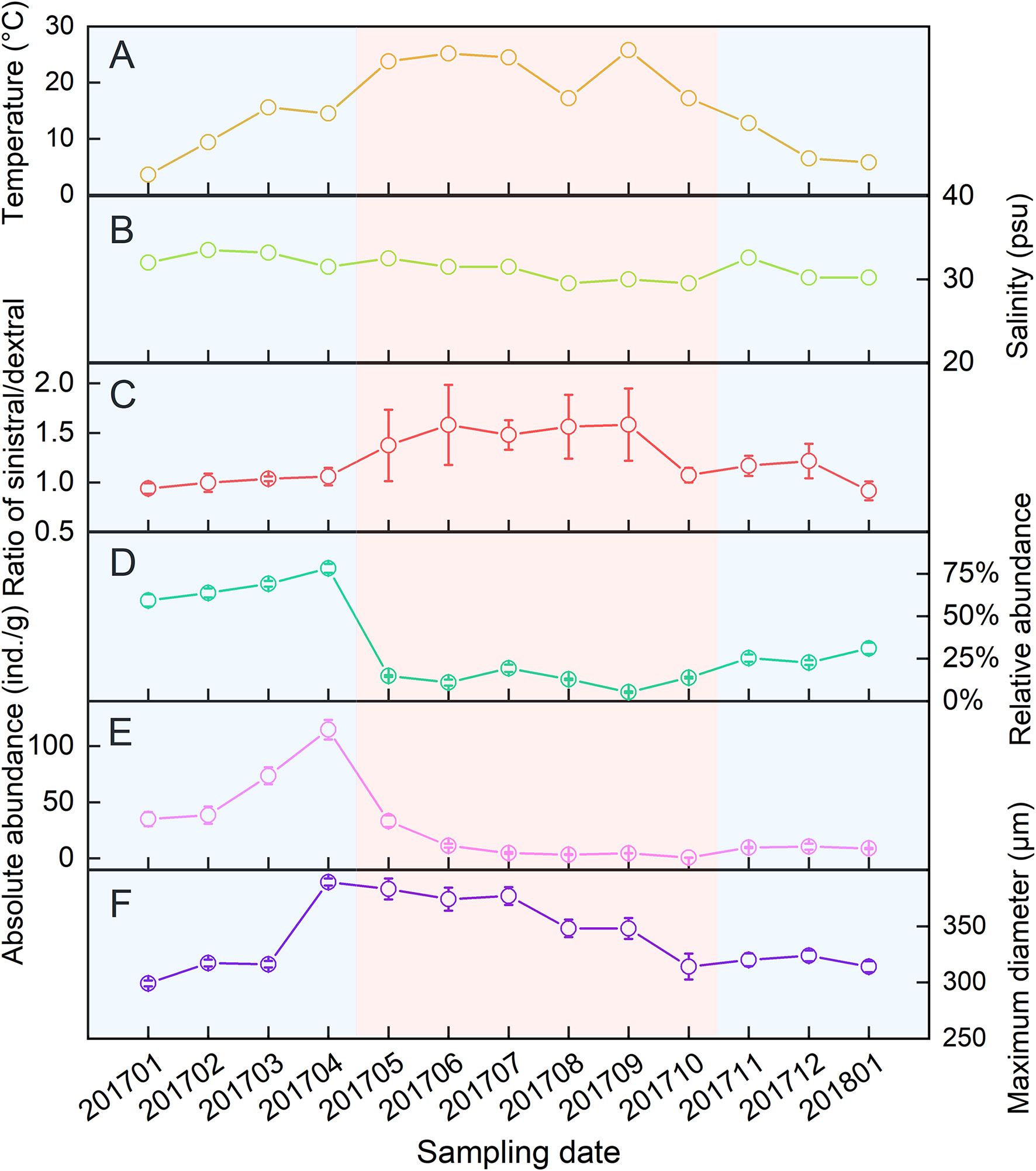
Figure 2 Variations of temperature (A), salinity (B), ratio of sinistral/dextral (C), abundance (D, E) and size (F) in A. aomoriensis among 13-months’ survey.
The absolute and relative abundance of A. aomoriensis were calculated respectively and both of them increased from January to April and then decreased until December (Figure 2D, E). The maximum diameter of each A. aomoriensis was also measured and showed a similar trend with the size (Figure 2F).
Statistical analysis showed no significant effects of temperature (F = 1.17, p = 0.3521) and salinity (F = 1.12, p = 0.3810) on the ratio of sinistral/dextral (Table 1). We conducted Pearson’s correlation analysis and the results showed the ratio of sinistral/dextral was significantly positively correlated with temperature (r = 0.50173, p = 0.0011) but not with salinity (r = –0.19449, p = 0.2354) (Table 2). A significant linear regression between the ratio of sinistral/dextral (y) and temperature (x) was established (y = 0.0266x + 0.8165, R2 = 0.25, p = 0.0011) (Figure 3A).
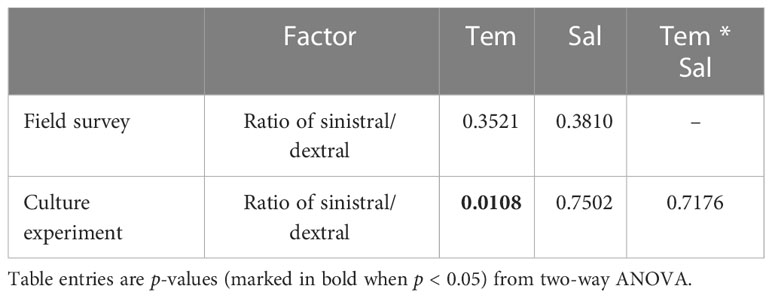
Table 1 p-values for effect of temperature (Tem) and salinity (Sal) on ratio of sinistral/dextral in A. aomoriensis from field survey and culture experiment.
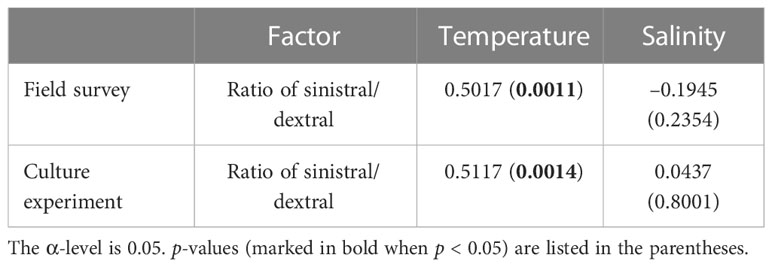
Table 2 Correlations (Pearson’s r values) between the ratio of sinistral/dextral and environmental factors.
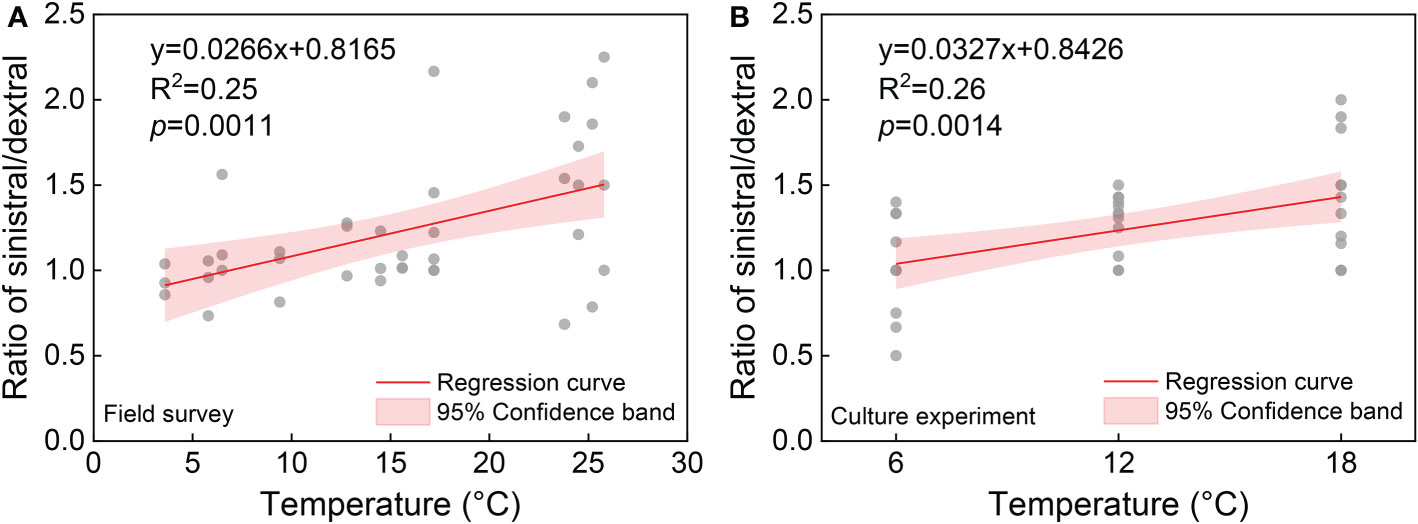
Figure 3 The relationship between ratio of sinistral/dextral and temperature from field survey (A) and culture experiment (B).
3.2 Culture experiment
A total of 554 live A. aomoriensis were obtained in 12 treatments. Among them, 316 were sinistral and 238 were dextral. Ratios of sinistral/dextral were 0.83 to 1.51. Ratio of sinistral/dextral showed an overall increasing trend with increasing temperature under the same salinity but showed no obvious pattern with the change of salinity (Figure 4).

Figure 4 Ratio of sinistral/dextral in A. aomoriensis under different temperature and salinity in the culture experiment.
Statistical analysis showed temperature had a significant effect on ratio of sinistral/dextral (F = 5.50, p = 0.0108), but not salinity (F = 0.41, p = 0.7502) (Table 1). In addition, interactive effect was not significant (F = 0.61, p = 0.7176). Tukey’s Studentized Range (HSD) test showed the ratio of sinistral/dextral at 18°C was significantly higher than that at 6°C (p < 0.05). There was no significant difference between 6 and 12°C and between 12 and 18°C. Tukey’s HSD test also showed no significant difference between any two salinity gradients. Results of Pearson’s correlation analysis showed the ratio of sinistral/dextral was significantly positively correlated with temperature (r = 0.51174, p = 0.0014) but not with salinity (r = 0.04373, p = 0.8001) (Table 2). A significant linear regression between the ratio of sinistral/dextral (y) and temperature (x) was established (y = 0.0327x + 0.8426, R2 = 0.26, p = 0.0014 < 0.05) (Figure 3B).
4 Discussion
4.1 Effects of temperature and salinity on the coiling direction in Ammonia aomoriensis
Temperature and salinity are important environmental factors, which have a vital effect on growth, reproduction, calcification, respiration of foraminifera. Foraminifedra are sensitive to the change in temperature and salinity, and foraminifera respond rapidly in their species composition, chemical element composition and morphology (Haynert and Schönfeld, 2014; Dong et al., 2019; Hauzer et al., 2021). Though the coiling direction as a proxy for paleoenvironment (especially the temperature) has been applied, there is no specific study on the effects of environmental factors on the coiling direction of living foraminifera. This work focuses on the effects of temperature and salinity on the coiling direction in A. aomoriensis using both field survey and culture experiment.
Annual field study can provide the most realistic data about the response of ratio direction in A. aomoriensis to natural environmental changes. However, environmental factors in natural habitats are not independent and the measured and other unknown factors will all have impacts on the observed result. It is difficult to determine the effect of a particular factor through field survey. Therefore, the culture experiment method is a good solution, in which only the studied factor changes and other factors are controlled. In this study, field survey results showed a significant correlation between coiling direction and temperature but ANOVA analysis showed no significant effect of temperature on coiling direction. This could be because other unknown environmental factors interacted simultaneously. The culture experiment results showed a highly consistent relationship with the field survey result (Figure 3) and verified the significant effect of temperature on the ratio of sinistral/dextral in A. aomoriensis. Combining the field survey and culture experiment, we confirmed that temperature significantly affected the coiling direction of A. aomoriensis in the study area and salinity did not. The higher temperature, the greater proportion of the sinistral individual, and vice versa. Previous studies showed that sinistral populations preferred cold waters in many foraminiferal species, while our study showed the opposite result. We thought that this might be species-specific and more studies on genus Ammonia were needed in the future.
4.2 The potential of coiling direction as a paleoceanographic proxy
The coiling direction is a distinct characteristic in trochospiral foraminifera. Compared to acquiring other shell characteristics (such as tubercule, aperture, proloculus size, chamber numbers, pore density and size, number of chambers, deformation and weight) that need professional knowledge of taxonomy and instruments with high resolution, the coiling direction of the shell is easily identified under normal magnification and the acquisition of coiling direction data is low cost in time and financial resource (Desmares et al., 2016).
The usefulness and reliability of coiling directing remain controversial because different studies showed inconsistent conclusions. However, the change of coiling direction universally occurred in the sediments from different regions and at different intervals in geologic time (Jenkins, 1967; Saito, 1976). Some studies have applied the variation of coiling direction in palaeoecological and palaeoclimatic reconstruction (Ericson, 1959; Galeotti and Coccioni, 2002). Therefore, we think the coiling direction of foraminifera is useful and reliable. It is worth noting that the geographical region and foraminiferal species need full consideration when applying the established coiling direction relationship. For this study, the correlation between coiling ratio and temperature is suited for A. aomoriensis in Yellow Sea and it may take more research work to revise before it can be used in other regions.
In the early studies, only the dead foraminiferal shells in sediments were studied for the coiling direction (Ericson, 1959; Herman, 1972). Then it was thought that environment determined the coiling direction to some extent. As the research progressed, some inconsistent and unexplained results emerged. Especially with the development in molecular biology, there was a growing opinion that coiling direction was controlled by gene (Norris and Nishi, 2001; Ujiié et al., 2010; Ujiié and Asami, 2014). Darling et al. (2006) provided genetic evidence to demonstrate the differently coiled specimens in Neogloboquadrina pachyderma belonged to different species. This prompted suspicion to the previous studies and there might occur cryptic species within the same species formerly thought (Linshy et al., 2007; Desmares et al., 2016; Luciani et al., 2021).
In terms of the possible genotypic differences in A. aomoriensis, we have performed molecular identification on two types of specimens with different coiling directions. Gene sequencing results showed sinistral and dextral specimens were same species (Lei et al., 2017). Our result showed that the expression of genetic information associated with coiling direction might be influenced by environment. Despite the significant correlation between coiling ratio and temperature, we can’t validate or disprove genetic determinism. More research work, especially transcriptomics, is needed to explore the control mechanism of coiling direction in foraminifera.
In this study, the reproduction of A. aomoriensis couldn’t be analyzed directly because we couldn’t identify individuals that newly born in the experiment. According to previous field survey data in the study area, A. aomoriensis reproduced twice a year (about January-February and July-August, respectively). We collected the benthic foraminifera samples in July and acclimated them for a month before the formal culture experiment. This made the benthic foraminiferal community stable and to some extent ensured that the analyzed specimen in the culture experiment were newly born individuals under the corresponding experimental conditions. In addition, the growth rate of A. aomoriensis was ~ 2 μm/day and the newly born individuals could grow to > 150 μm during the culture experiment (10 weeks). Therefore, the large benthic foraminiferal specimen (> 150 μm) could provide the reliable data of coiling direction in this study. The small size group (< 150 μm) usually included some small or juvenile species, which was difficult and time consuming to identify. We omitted the small size benthic foraminiferal specimen because we thought the large benthic foraminifera represented the same information. In present study, the relatively small individuals (63–150 μm) were preserved for future studies. Even so, the final analyzed sample inevitably contained the individuals from the initial benthic foraminiferal community from the study area. However, these effects could be ignored and didn’t affect the conclusions.
4.3 Evolutionary significance of the variation of coiling direction in foraminifera
In this study, A. aomoriensis preferred sinistral coiling direction and the ratio of sinistral/dextral was usually more than 1. Previous field studies also showed similar results in the Ammonia genus (Lei et al., 2017; Schönfeld et al., 2021). An interesting relationship between the ratio of sinistral/dextral and the size and abundance of A. aomoriensis was found in this study (Table 3). That is the larger the individual, the more sinistral. A similar trend was also reported in planktic foraminifera (Thiede, 1971). The change of size in foraminifera usually reflects the subjected stress and has certain selectivity in evolution (Keller and Abramovich, 2009). The previous study showed that the larger foraminifera was susceptible to extinction (Cotton and Pearson, 2011; Song et al., 2011). Norris and Nishi (2001) also found that the planktic species with a preference for sinistral or dextral coiling were more vulnerable to extinction than species with random coiling direction. There was an ecological separation between species with different coiling directions (Pearson and Penny, 2021). The change of coiling ratio might reflect the competitive relationship for different ecological habitats between species with sinistral and dextral coiling.
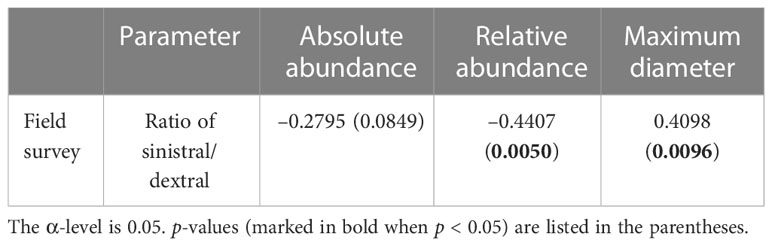
Table 3 Correlations (Pearson’s r values) between the ratio of sinistral/dextral and other biotic parameters of A. aomoriensis in the field survey.
Previous studies also suggested that coiling direction correlated to the reproduction in foraminifera (Linshy et al., 2007; Khare et al., 2012; Davis et al., 2020). In this study, we thought the variation of abundance and size could represent two types of reproductive strategies. In the cold seasons (January to April, November to December), A. aomoriensis was smaller in size and more in abundance (r-selection in reproductive strategy), and it was larger and less in warm seasons (May to October) (K-selection in reproductive strategy) (Figure 2). The low value of coiling ratio (close to 1) coinciding with smaller individuals showed an adaptive response to the stressed condition (low temperature) and might be a synergetic mechanism to reduce the probability of extinction in evolution. In contrast, coiling direction might be selected and fixed in a suitable condition, which is reflected in the increase of sinistral coiling in present study. This work offers a new perspective on the variation of coiling direction in foraminifera in sight of environmental adaptation and evolution.
5 Conclusions
In this work, we studied the coiling direction of benthic foraminifera combining the field survey and culture experiment. A. aomoriensis preferred sinistral under high temperatures. The ratio of sinistral/dextral in A. aomoriensis was significantly positively correlated with temperature, but had no evident relationship with salinity. Significant correlations between coiling direction and size and abundance of A. aomoriensis were found. We propose that the variation of coiling direction in foraminifera may be an adaptive response to environmental changes and a synergetic mechanism in evolution. We confirm the coiling direction in A. aomoriensis is a useful and reliable proxy for temperature and the relationships can be used as the environmental indicator and paleoceanographic reconstruction in the study area.
Data availability statement
The original contributions presented in the study are included in the article. Further inquiries can be directed to the corresponding author.
Author contributions
SD: Conceptualization, Methodology, Formal analysis, Investigation, Data curation, Writing – original draft preparation, Funding acquisition. YL: Conceptualization, Supervision, Writing – review and editing, Project administration, Resources, Funding acquisition. TW: Data curation, Validation, Software. ML: Investigation, Data curation, Software. All authors contributed to the article and approved the submitted version.
Funding
This work received financial supports from the following projects: the National Natural Science Foundation of China (No. U1906211), Laoshan Laboratory (No. LSKJ202204200), the Strategic Priority Research Program of the Chinese Academy of Sciences (No. XDB42000000), Wenhai Program of Laoshan Laboratory (No. LSKJ202204900) and Natural Science Foundation of Shandong Province (Grant Nos. ZR2021QD004).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Billups K., Hudson C., Kunz H., Rew I. (2016). Exploring Globorotalia truncatulinoides coiling ratios as a proxy for subtropical gyre dynamics in the northwestern Atlantic ocean during late pleistocene ice ages. Paleoceanography 31 (5), 553–563. doi: 10.1002/2016PA002927
Boehnert S., Birkelund A. R., Schmiedl G., Kuhnert H., Kuhn G., Hass H. C., et al. (2020). Test deformation and chemistry of foraminifera as response to anthropogenic heavy metal input. Mar. pollut. Bull. 155, 111112. doi: 10.1016/j.marpolbul.2020.111112
Cotton L. J., Pearson P. N. (2011). Extinction of larger benthic foraminifera at the Eocene/Oligocene boundary. Palaeogeogr. Palaeoclimatol. Palaeoecol. 311 (3), 281–296. doi: 10.1016/j.palaeo.2011.09.008
Darling K. F., Kucera M., Kroon D., Wade C. M. (2006). A resolution for the coiling direction paradox in Neogloboquadrina pachyderma. Paleoceanography 21 (2), PA2011. doi: 10.1029/2005PA001189
Davis C. V., Livsey C. M., Palmer H. M., Hull P. M., Thomas E., Hill T. M., et al. (2020). Extensive morphological variability in asexually produced planktic foraminifera. Sci. Adv. 6 (28), eabb8930. doi: 10.1126/sciadv.abb8930
Desmares D., Crognier N., Bardin J., Testé M., Beaudoin B., Grosheny D. (2016). A new proxy for Cretaceous paleoceanographic and paleoclimatic reconstructions: Coiling direction changes in the planktonic foraminifera Muricohedbergella delrioensis. Palaeogeography Palaeoclimatology Palaeoecol. 445, 8–17. doi: 10.1016/j.palaeo.2015.12.021
Dong S., Lei Y., Li T., Jian Z. (2019). Responses of benthic foraminifera to changes of temperature and salinity: Results from a laboratory culture experiment. Sci. China Earth Sci. 62 (2), 459–472. doi: 10.1007/s11430-017-9269-3
Dong S., Lei Y., Li T., Jian Z. (2020). Response of benthic foraminifera to pH changes: Community structure and morphological transformation studies from a microcosm experiment. Mar. Micropaleontology 156, 101819. doi: 10.1016/j.marmicro.2019.101819
Ericson D. B. (1959). Coiling direction of Globigerina pachyderma as a climatic index. Science 130 (3369), 219–220. doi: 10.1126/science.130.3369.219
Galeotti S., Coccioni R. (2002). Changes in coiling direction of Cibicidoides pseudoacutus (Nakkady) across the Cretaceous–tertiary boundary of Tunisia: palaeoecological and biostratigraphic implications. Palaeogeography Palaeoclimatology Palaeoecol. 178 (3), 197–210. doi: 10.1016/S0031-0182(01)00396-0
Hallock P., Rosenkrands Larsen A. (1979). Coiling direction in Amphistegina. Mar. Micropaleontology 4, 33–44. doi: 10.1016/0377-8398(79)90004-5
Hauzer H., Evans D., Müller W., Rosenthal Y., Erez J. (2021). Salinity effect on trace element incorporation in cultured shells of the large benthic foraminifer Operculina ammonoides. Paleoceanography Paleoclimatology 36 (6), e2021PA004218. doi: 10.1029/2021PA004218
Haynert K., Schönfeld J. (2014). Impact of changing carbonate chemistry, temperature, and salinity on growth and test degradation of the benthic foraminifer Ammonia aomoriensis. J. Foraminiferal Res. 44 (2), 76–89. doi: 10.2113/gsjfr.44.2.76
Herman Y. (1972). Globorotalia truncatulinoides: a palaeo-oceanographic indicator. Nature 238 (5364), 394–396. doi: 10.1038/238394a0
Jenkins D. G. (1967). Recent distribution, origin, and coiling ratio changes in globorotalia pachyderma (Ehrenberg). Micropaleontology 13 (2), 195–203. doi: 10.2307/1484670
Keller G., Abramovich S. (2009). Lilliput Effect in late maastrichtian planktic foraminifera: Response to environmental stress. Palaeogeography Palaeoclimatology Palaeoecol. 284 (1), 47–62. doi: 10.1016/j.palaeo.2009.08.029
Khare N., Mazumder A., Govil P. (2012). Do changes in coiling directions in planktonic foraminifera correspond to dimorphic reproduction? Oceanology 52 (3), 364–371. doi: 10.1134/s0001437012030058
Lei Y., Li T., Nigam R., Holzmann M., Lyu M. (2017). Environmental significance of morphological variations in the foraminifer Ammonia aomoriensis (Asan) and its molecular identification: A study from the yellow Sea and East China Sea, PR China. Palaeogeography Palaeoclimatology Palaeoecol. 483, 49–57. doi: 10.1016/j.palaeo.2016.05.010
Li M., Lei Y., Li T., Dong S. (2020). Response of intertidal foraminiferal assemblages to salinity changes in a laboratory culture experiment. J. Foraminiferal Res. 50 (4), 319–329. doi: 10.2113/gsjfr.50.4.319
Linshy V. N., Rana S. S., Kurtarkar S., Saraswat R., Nigam R. (2007). Appraisal of laboratory culture experiments on benthic foraminifera to assess/develop paleoceanographic proxies. Indian J. Mar. Sci. 36 (4), 301–321.
Luciani V., D’Onofrio R., Dickens G. R., Wade B. S. (2021). Dextral to sinistral coiling switch in planktic foraminifer Morozovella during the early Eocene climatic optimum. Global Planetary Change 206, 103634. doi: 10.1016/j.gloplacha.2021.103634
Nigam R., Khare N. (1992). The reciprocity between coiling direction and dimorphic reproduction in benthic foraminifera. J. Micropalaeontology 11 (2), 221–228. doi: 10.1144/jm.11.2.221
Norris R. D., Nishi H. (2001). Evolutionary trends in coiling of tropical paleogene planktic foraminifera. Paleobiology 27 (2), 327–347. doi: 10.1666/0094-8373(2001)027<0327:ETICOT>2.0.CO;2
Pearson P. N., Penny L. (2021). Coiling directions in the planktonic foraminifer Pulleniatina: A complex eco-evolutionary dynamic spanning millions of years. PloS One 16 (4), e0249113. doi: 10.1371/journal.pone.0249113
Romano E., Bergamin L., Di Bella L., Frezza V., Pierfranceschi G., Marassich A., et al. (2021). Benthic foraminifera as environmental indicators in extreme environments: The marine cave of bue Marino (Sardinia, Italy). Ecol. Indic. 120, 106977. doi: 10.1016/j.ecolind.2020.106977
Saito T. (1976). Geologic significance of coiling direction in the planktonic foraminifera Pulleniatina. Geology 4 (5), 305–309. doi: 10.1130/0091-7613(1976)4<305:GSOCDI>2.0.CO;2
Saraswat R. (2015). Non-destructive foraminiferal paleoclimatic proxies: a brief insight. Proc. Indian Natl. Sci. Acad. 81 (2), 381–395. doi: 10.16943/ptinsa/2015/v81i2/48094
Schönfeld J., Beccari V., Schmidt S., Spezzaferri S. (2021). Biometry and taxonomy of Adriatic Ammonia species from bellaria–igea Marina (Italy). J. Micropalaeontology 40 (2), 195–223. doi: 10.5194/jm-40-195-2021
Song H., Tong J., Chen Z. Q. (2011). Evolutionary dynamics of the Permian–Triassic foraminifer size: Evidence for Lilliput effect in the end-Permian mass extinction and its aftermath. Palaeogeogr. Palaeoclimatol. Palaeoecol. 308 (1-2), 98–110. doi: 10.1016/j.palaeo.2010.10.036
Thiede J. (1971). Variations in coiling ratios of Holocene planktonic foraminifera. Deep Sea Res. Oceanographic Abstracts 18 (8), 823–831. doi: 10.1016/0011-7471(71)90049-0
Ujiié Y., Asami T. (2014). Temperature is not responsible for left-right reversal in pelagic unicellular zooplanktons. J. Zoology 293 (1), 16–24. doi: 10.1111/jzo.12095
Keywords: Ammonia aomoriensis, sinistral, dextral, salinity, paleoceanographic reconstruction
Citation: Dong S, Lei Y, Wu T and Li M (2023) New evidence for coiling direction of benthic foraminifera as a temperature proxy. Front. Mar. Sci. 10:1122189. doi: 10.3389/fmars.2023.1122189
Received: 12 December 2022; Accepted: 10 February 2023;
Published: 20 February 2023.
Edited by:
Haifeng Gu, State Oceanic Administration, ChinaReviewed by:
Petra Heinz, University of Vienna, AustriaBaichuan Duan, Ministry of Natural Resources, China
Copyright © 2023 Dong, Lei, Wu and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yanli Lei, leiyanli@qdio.ac.cn
 Shuaishuai Dong1
Shuaishuai Dong1  Yanli Lei
Yanli Lei Tianzhen Wu
Tianzhen Wu