The Occurrence and Distribution of Benthic Foraminifera in Tropical Waters Along the Strait of Malacca
- 1Faculty of Science and Marine Environment, University of Malaysia, Terengganu, Kuala Nerus, Malaysia
- 2Paleoceanography Research Interest Group, University of Malaysia, Terengganu, Kuala Nerus, Malaysia
- 3Institute of Oceanography and Environment, University of Malaysia, Terengganu, Kuala Nerus, Malaysia
- 4Department of Geology, University of Madras, Chennai, India
- 5Coastal Management and Oceanography Research Centre, National Hydraulic Research Institute of Malaysia, Sri Kembangan, Malaysia
Foraminifera are shelled single-celled protists that are found in all marine environments. Benthic foraminifera either live in sediments or attach to surfaces on the seafloor. Understanding the distribution and ecological response of benthic foraminifera is crucial, as they can indicate past and current ocean conditions. However, the benthic foraminifera distribution along the busy Strait of Malacca, which connects the Indian Ocean (north) to the Java Sea (south), is undersampled. In this study, we collected 24 surface samples from the northern Strait of Malacca to understand the distribution of foraminifera assemblages in shallow tropical waters. A total of 49 species of benthic foraminifera were identified. Calcareous hyaline species dominated the assemblages, with an extremely low occurrence of calcareous porcelaneous species. The common calcareous hyaline taxa were Asterorotalia pulchella, Pseudorotalia schroeteriana, Discorbinella bertheloti, Ammonia tepida, and Heterolepa praecincta. Cluster analysis categorised the foraminiferal assemblages into three major groups. The first cluster (Group A) consisted of a more diverse assemblage of hyaline and agglutinated species that inhabited a mean water depth of 45 m. The second cluster represented a population that inhabited deeper water environments (average water depth of 59 m). Finally, the third cluster (Group C) consisted of a foraminifera assemblage that inhabited shallow coastal environments (average depth of 22 m) with higher organic matter enrichment. The multivariate canonical correspondence analysis (CCA) showed that the foraminiferal assemblages reflected the shallow to deep water transition in the Malacca Strait. Water depth, which defines the depositional environment, had a greater influence on foraminifera distribution here than organic matter and salinity.
Introduction
The Strait of Malacca is a shallow water passage between peninsular Malaysia and Sumatra that connects the Indian and Pacific Oceans. It is also a global shipping marine route, with more than 120,000 ships passing through the straits annually. The narrow Strait of Malacca was a savannah corridor during the Last Glacial Maximum when the sea level was much lower than the present day. Today, the shallow strait receives freshwater runoff from both peninsular Malaysia and Sumatra, resulting in more hyposaline environments closer to the coastline. These characteristics have endowed the region with abundant non-renewable and renewable resources, including profitable coastal ecosystems, aquaculture, coastal tourism, extensive capture fisheries, valuable natural gas reserves, and mining (Evers and Gerke, 2006). However, the region is also repeatedly threatened by the overexploitation of living resources and the introduction of harmful domestic and industrial waste (Chua et al., 2000). With growing maritime and coastal activities, key stakeholders are under increasing pressure to minimise the future impact on environmental services along the Malacca Strait.
Foraminifera can help monitor the environmental status of both sandy and soft-bottom marine ecosystems (Hallock et al., 2003; Sreenivasulu et al., 2019; Sousa et al., 2020). Due to their widespread distribution, modern benthic foraminifera have been used to assess the ecological health of lagoon environments (Culver et al., 2012; Bouchet et al., 2018), estuaries (Nagendra and Reddy, 2019; Sreenivasulu et al., 2019), coral reefs (Nurruhwati et al., 2020; Prazeres et al., 2020), and modern harbours (Dijkstra et al., 2017). Moreover, foraminiferal assemblages are influenced by their immediate environment and have often served as modern and past analogues to characterise paleoenvironments (Rao et al., 2013; Benito et al., 2016; Minhat et al., 2016; Kemp et al., 2018). Therefore, documenting and understanding the regional distribution of foraminifera are essential. This study documented the distribution of benthic foraminifera species in the Malacca Strait. We also assessed the dominant environmental factors influencing benthic foraminifera distribution in one of the world’s busiest shipping lanes.
Study Area
The northern region of the Strait connects with the deeper Andaman Sea and is more saline (>34 PSU) than the middle and southern sectors of the strait (Amiruddin et al., 2011). Major rivers from Sumatra and peninsular Malaysia discharge approximately 19 × 1010 m3 of freshwater annually to both sites of the strait, creating hyposaline conditions along most parts of the strait (Hii et al., 2006; Amiruddin et al., 2011). Numerous ecosystems have been reported along the strait, including mangroves, coral reefs, seagrass, and extended mudflats (Yasin et al., 2019). Two tropical monsoon seasons influence the weather along the Strait of Malacca. These monsoon winds create wetter conditions during the northeast monsoon period and drier conditions during the southwest monsoon period (Hii et al., 2006; Amiruddin et al., 2011).
Sample Collection
A total of 24 surface sediment samples were collected using a Smith McIntyre grab onboard the UMT RV Discovery (15 × 5 m) during the UMT Scientific Expedition Voyage LIMA’19 from 13 to 23 March, 2019. These samples were collected along the northern regions of the Malacca Strait within Malaysian waters (Figure 1). We collected sub-samples from the bulk sediments for both sediment (~40 g) and foraminifera analyses (10 cm3; 10 cm2 × 1 cm; Scott and Medioli, 1980; Minhat et al., 2020). Ethanol solution (>70%) was used to preserve the foraminifera samples. We recorded the coordinates of each station, and water depth and bottom water salinity were measured using a Conductivity, Temperature, Depth (CTD) castaway profiler. We obtained salinity records for all stations except W35, as the depth was too shallow for CTD deployment.
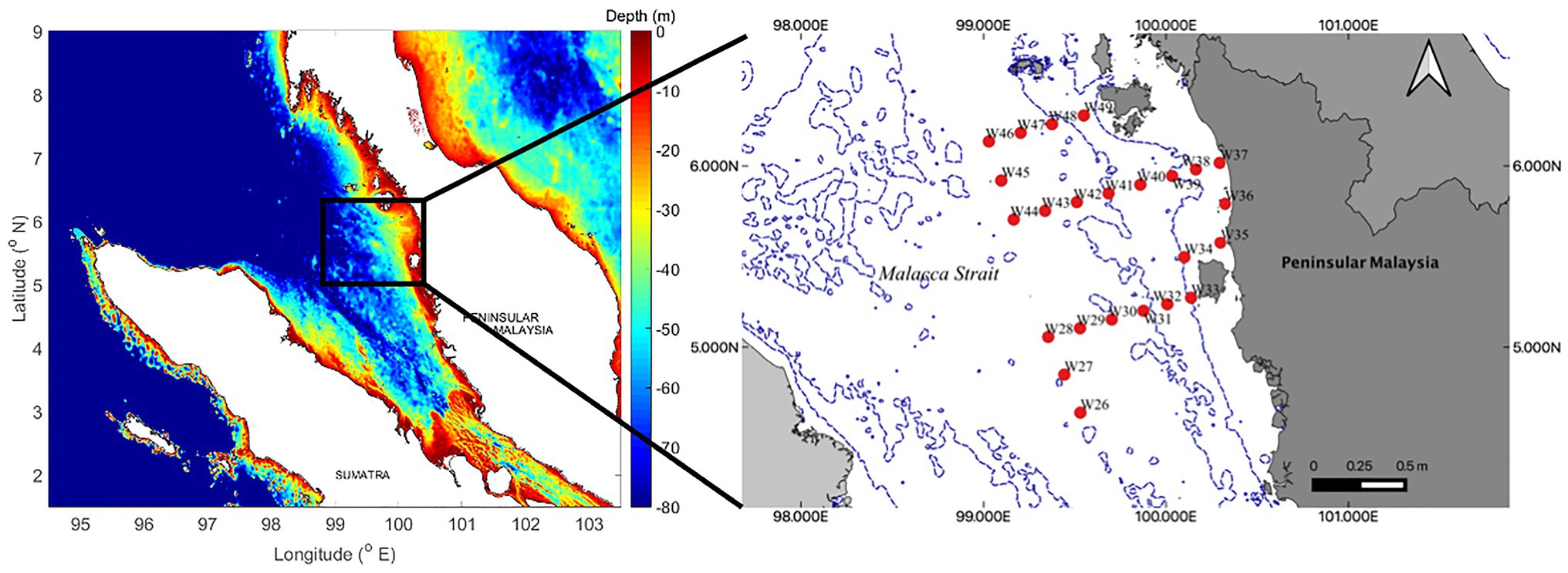
Figure 1. Twenty-four sampling stations located in the northern sector of the Malacca Strait. All samples were collected within Malaysian waters.
Laboratory Analysis
Foraminifera Analyses
Sediment samples were passed through 63 μm sieves under running tap water (Schönfeld et al., 2012). The residues on the sieve were decanted into labelled boats and left to dry overnight in an oven at 50°C. The dried foraminifera samples were then transferred and stored in plastic bags until analysed.
Prior to sorting, the samples were equally divided into aliquots using a microsplitter. A total of 300 foraminifera specimens were randomly picked from a gridded picking tray using a fine artist brush and then sorted on micropalaeontology slides. During the picking process, foraminiferal tests that were discoloured, broken, or poorly preserved (Yordanova and Hohenegger, 2002) were excluded. The sorted specimens were identified at the species level based on Loeblich and Tappan (1988, 1994) and regional taxonomic references (Szarek, 2001; Martin et al., 2018; Minhat et al., 2020).
Sediment and Organic Matter Analyses
The sediment grain size was determined via the dry sieving method (Folk, 1980), and the organic matter composition was determined via the loss-on-ignition method (Heiri et al., 2001).
Computation of Diversity Indices and Statistical Analyses
To compare our results with previous work by Minhat et al. (2020), Fisher’s alpha diversity and Pielou’s evenness indices (Pielou, 1969) were calculated using PRIMER v.6 software (Clarke and Gorley, 2006). To avoid using reworked specimens, only foraminiferal taxa with a relative abundance of >2% in atleast one sample were used to determine the foraminiferal zonation and fauna-environment relationship. The Q-mode hierarchical cluster analysis based on the Euclidean distance similarity measure was used to understand the biozonation of foraminiferal assemblages (Mello and Buzas, 1968; Culver et al., 2012; Azmi et al., 2020). Multivariate canonical correspondence analysis (CCA) was calculated using XLSTAT software to determine the influence of environmental variables, water depth, and sediment characteristics on foraminiferal assemblages. The CCA was performed on all 49 foraminifera species with >2% relative abundance.
Results
Environmental Conditions Along the Northern Sector of the Malacca Strait
The water depth of the study area ranged from 9 to 86 m, with recorded salinities of 32–34 PSU (Table 1; Figure 2A). The average proportion of organic matter in this study was 7.1%, with the highest organic matter content recorded at station W36 (17%; Figure 2B). Most stations were predominantly sandy, with a sand composition of 97% at W30. Moreover, stations with deeper water depths (>60 m) had relatively high mud compositions (mud >60%; Figures 2C,D).
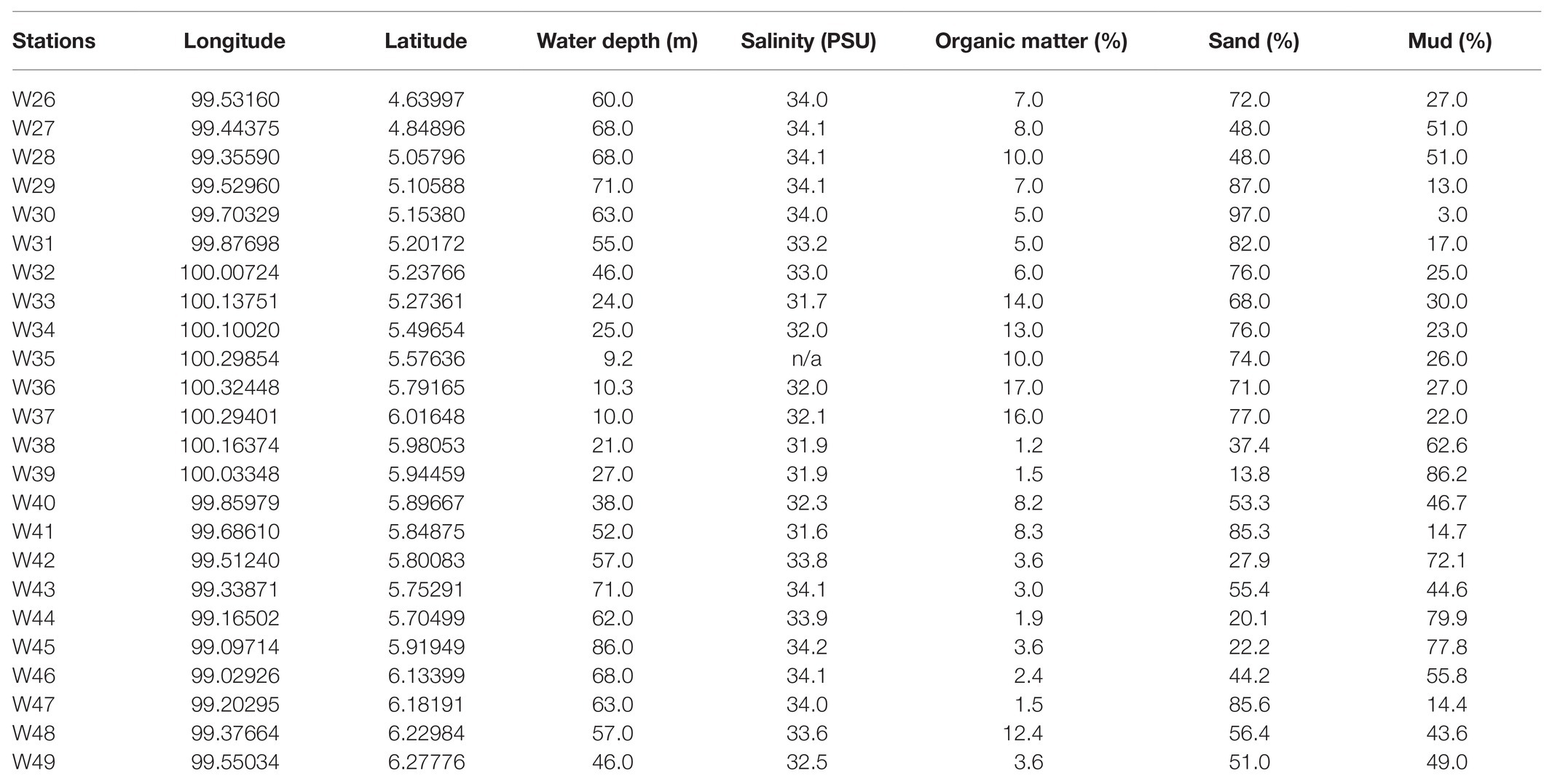
Table 1. List of 24 stations within the Malacca Straits with station coordinates, water depth, salinity, organic matter, and sand and mud compositions.
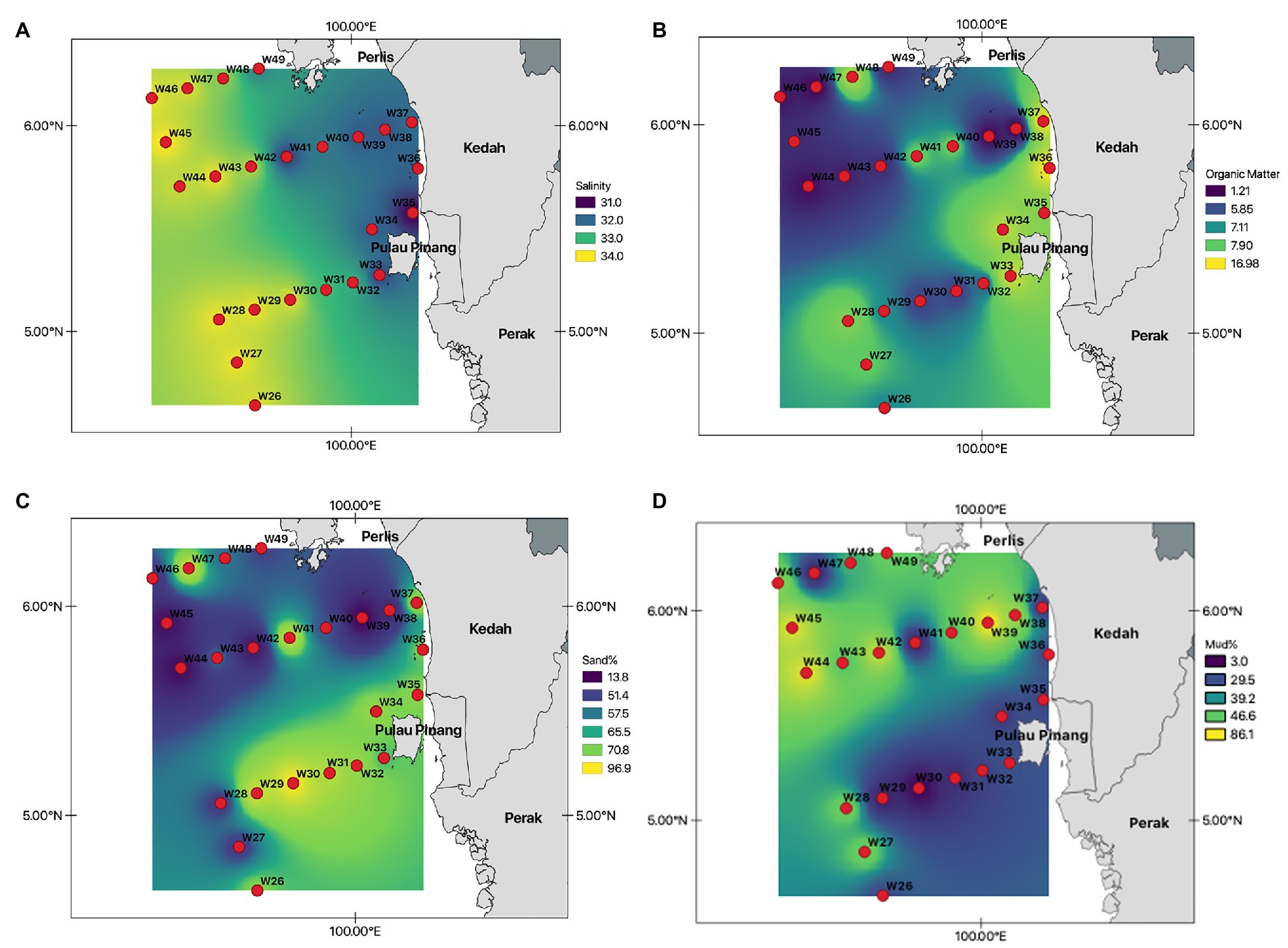
Figure 2. Selected environmental variables recorded at all 24 stations within the northern sector of Malacca Straits. (A) – Salinity (PSU); (B) – Organic matter (%); (C) – Sand (%); and (D) – Mud (%).
Foraminiferal Assemblages
A total of 90 foraminifera species were identified from the 24 stations in the northern region of the Strait of Malacca. However, only 49 species had >2% relative abundance, and 20 species had >10% relative abundance in atleast one station (Supplementary Table S1). On average, the calcareous hyaline (86%) group dominated the total foraminifera assemblages in the study area. In comparison, the agglutinated group and porcelaneous group accounted for 13 and <1% of the total assemblages, respectively (Table 2). The common calcareous hyaline species identified were Asterorotalia pulchella, Pseudorotalia schroeteriana, Discorbinella bertheloti, Ammonia tepida, and Heterolepa praecincta (Figure 3). Of these, A. pulchella was recorded at all sampling stations and was therefore the most common species in the study area (Table 2). Moreover, four other species (P. schroeteriana, D. bertheloti, A. tepida, and H. praecincta) were recorded at more than 15 stations.
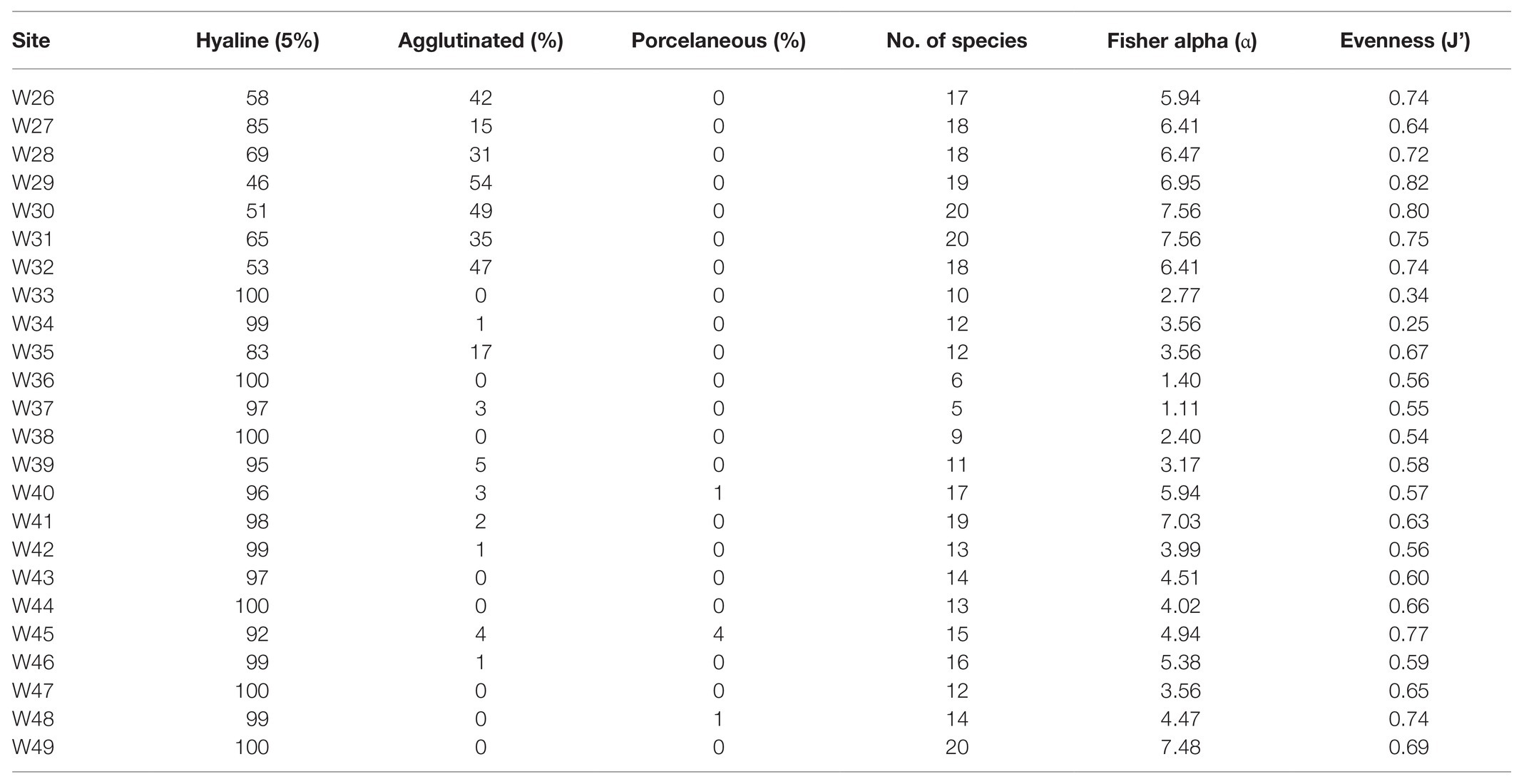
Table 2. Distribution of hyaline, agglutinated, and porcelaneous foraminifera groups within the Malacca Straits. The computed diversity indices and Fisher alpha (α) are also included.
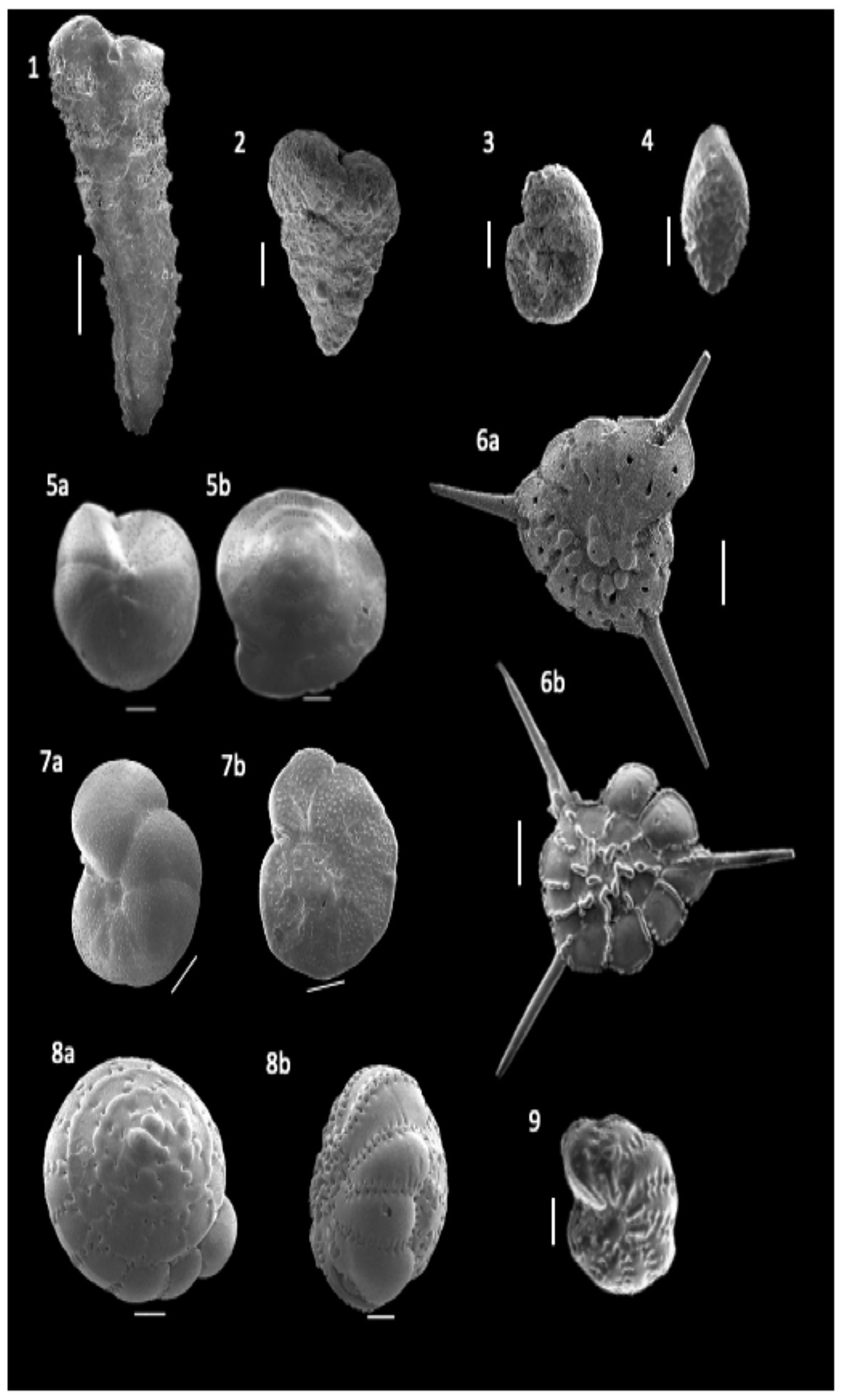
Figure 3. Scanning Electron Microscope images of among the benthic foraminifera identified from Malacca Straits. (1) Textularia fistula (magnification- x70; scale bar = 200 μm). (2) Textularia fistula (magnification- x150; scale bar = 100 μm). (3) Trochammina sp. 1 (magnification- x150; scale bar = 100 μm). (4) Bolivina glutinata (magnification- x250; scale bar = 100 μm). (5a) Heterolepa praecincta (magnification- x150; scale bar = 100 μm). (5b) Heterolepa praecincta (magnification- x140; scale bar = 100 μm). (6a) Asterorotalia pulchella (magnification- x70; scale bar = 200 μm). (6b) Asterorotalia pulchella (magnification- x85; scale bar = 200 μm). (7a) Discorbinella berteloti (magnification- x100; scale bar = 100 μm). (7b) Discorbinella berteloti (magnification- x180; scale bar = 100 μm). (8a) Pseudorotalia indopacifica (magnification- x60; scale bar = 200 μm). (8b) Pseudorotalia indopacifica (magnification- x55; scale bar = 200 μm). (9) Elphidium advenum (magnification- x200; scale bar = 100 μm).
The assemblage diversity, measured by Fisher’s alpha (α), ranged between 1.11 (W37) and 7.56 (W30 and W31), and the number of species present at each station was between S = 5 and S = 20 (Table 2). The species distribution was the most evenly distributed [Pielou evenness (J’) = 0.82] at W29 and the least evenly distributed (J’ = 0.25) at W34. The latter site was dominated by A. pulchella (76%).
Cluster Analysis
The Q-mode hierarchical cluster analysis based on the 24 stations produced three distinct groups (Figure 4). The first cluster (Group A) included samples from eight stations with an average water depth of 45 m. The foraminiferal assemblage within this group was dominated by A. pulchella (19%), followed by D. bertheloti (8%), and Elphidium advenum (7%). Agglutinated taxa, such as Agglutinella agglutinans (3%) and Textularia spp. (1–4%), were also relatively higher in Group A than in the other groups. The second cluster (Group B) contained half of the sampling stations that were generally located at deeper water depths (average water depth = 59 m). This group was co-dominated by A. pulchella (16%) and Bolivina glutinata (15%). Moreover, the porcelaneous species Quinqueloculina crassicarinata was only recorded in Group B. Finally, the third cluster (Group C) consisted of a foraminiferal assemblage from shallow coastal waters (average water depth = 23 m) and was dominated by three species: A. pulchella (27%), Ammonia convexa (26%), and A. tepida (21%). Agglutinated species were absent in Group C, except for Trochaminna sp. 1, which had very low abundance (1%).
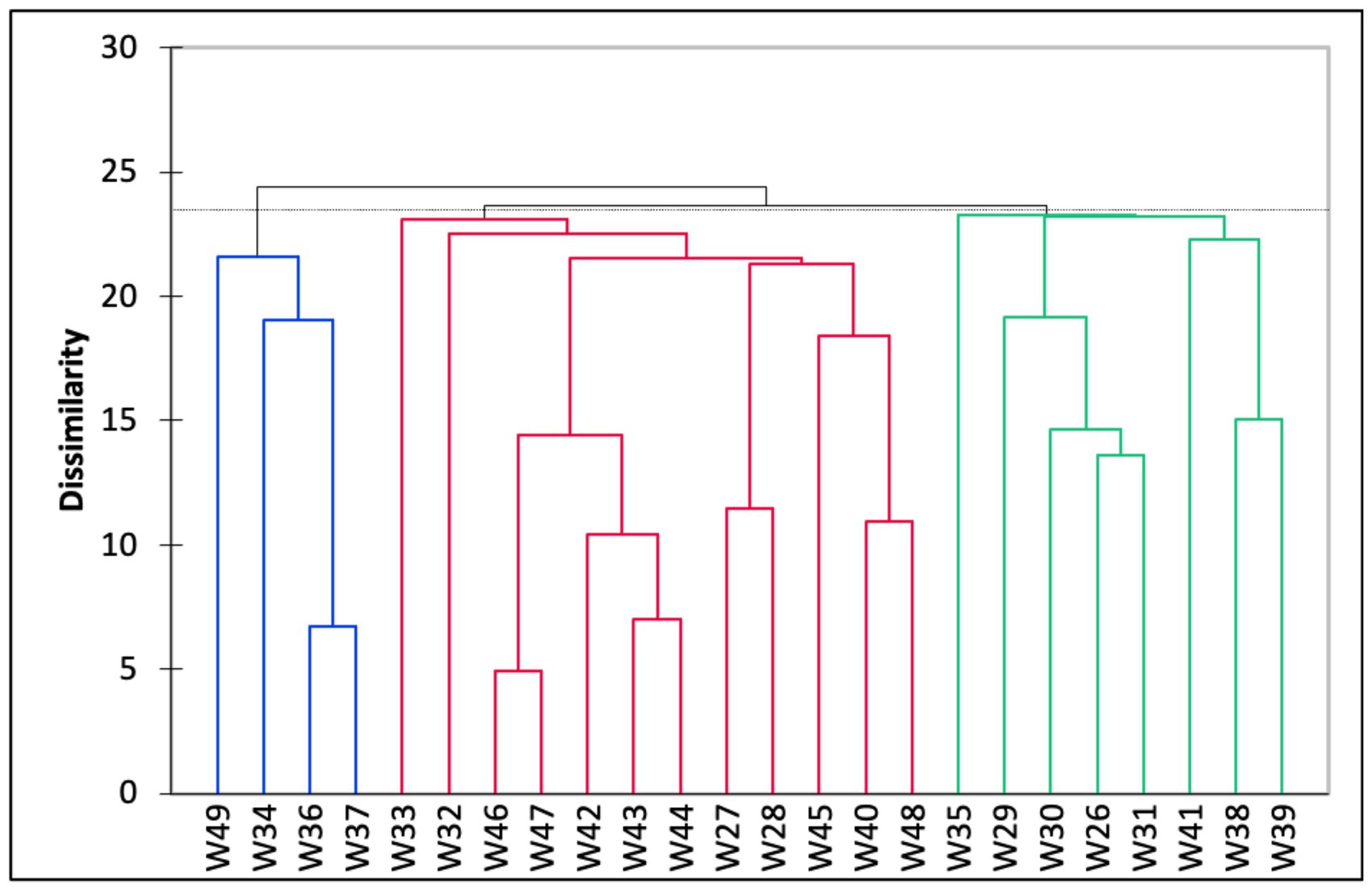
Figure 4. Dendrogram based on hierarchical cluster analysis on all 24 samples collected in the study area, Malacca Straits. The clusters were defined into three major clusters (i.e., Groups A–C) based on the dissimilarity between cluster groups.
Species-Environment Relationship
The total variance calculated by CCA between species and the environmental parameters was 70.1% for both axis-1 (eigenvalue: 0.61) and axis-2 (eigenvalue: 0.33; Figure 5). The results of the Monte Carlo permutation test (p < 0.0001; 500 permutation reduced model) suggest that these variables (i.e., water depth, salinity, organic matter content, and percentage of sand and mud) significantly influenced the foraminiferal distribution in the Malacca Strait. CCA axis-1 showed that water depth and organic matter content were the two most significant variables influencing foraminiferal distribution. This axis also reflects the shallow to deep water transition in the Malacca Strait.
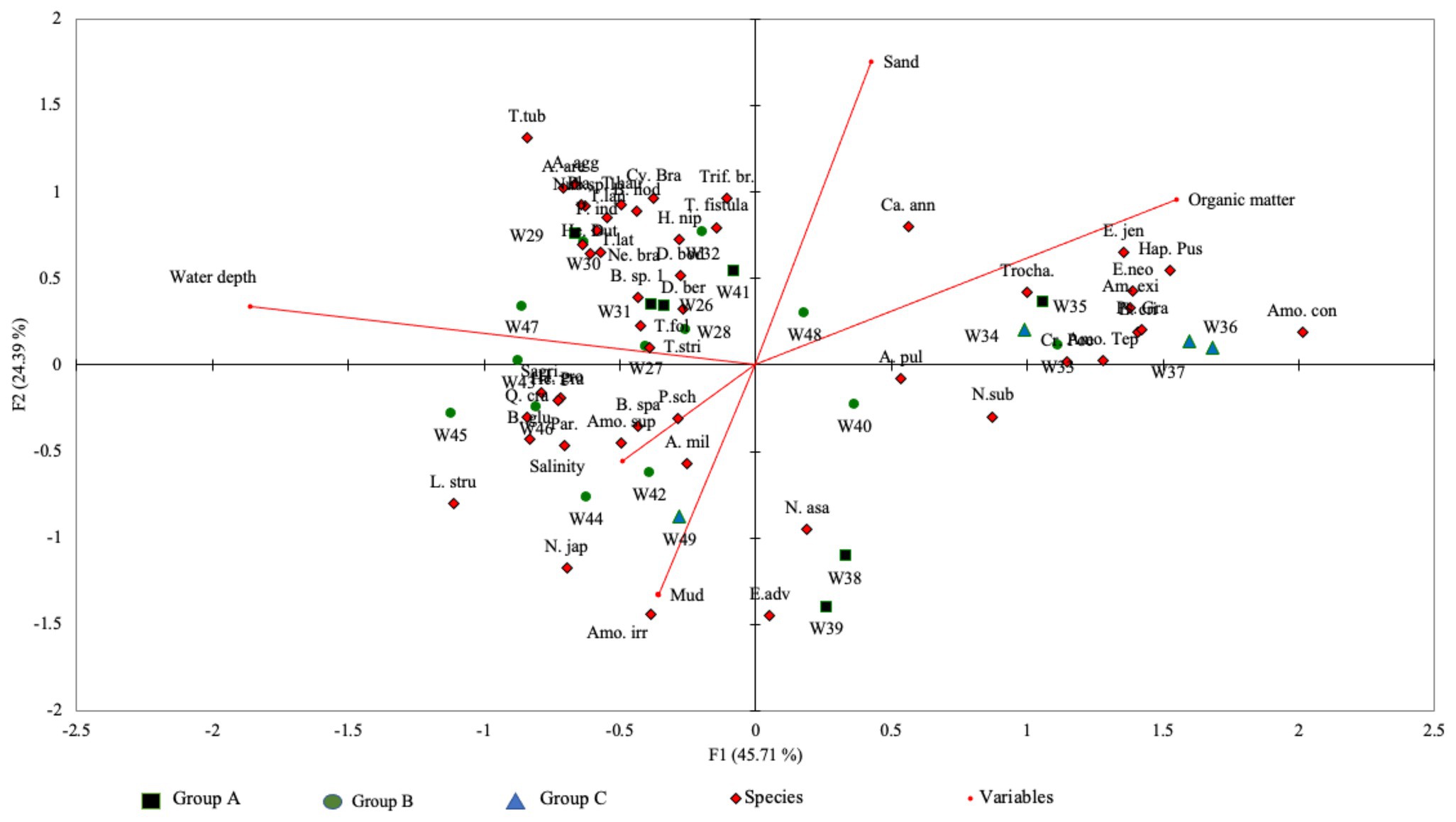
Figure 5. Canonical correspondence analysis (CCA) triplot based on species-environmental relationship along the Malacca Straits. The total variances of both axes were 70.1%.
Discussion
Species Composition
The most common species recorded at all stations was A. pulchella, with a relative abundance of 1–76%. This species, which was identified as Asterorotalia trispinosa by Panchang and Nigam (2012) along the Myanmar shelf, prefers low salinity and fine-grained sediment. In this study, the relative abundance of A. pulchella was >10% in stations with a salinity of 32 PSU or less. In addition, the relatively higher abundance of A. pulchella (>40%) in the Malacca Strait was associated with a water depth of 21–25 m. Pseudorotalia schroeteriana was also common in the Malacca Strait (present in 20 out of the 24 stations) but in low relative abundances (<10%). Moreover, the distribution of D. bertheloti was observed at 50–60 m water depth at relative abundances of >15%. A similar finding was reported by Azmi et al. (2020) in the southern region of the South China Sea, where D. bertheloti represented 20% of the foraminiferal assemblages at ~56 m depth. We, therefore, confirm that this species represents depths of 50–60 m in sandy tropical waters. Heterolepa praecincta is another common species that contributed >10% to the foraminiferal assemblages in deep-water environments (57–71 m water depth). Both D. bertheloti and H. praecincta were absent in samples from stations W36 and W37, where A. tepida (37–40%) appears to dominate. The higher organic matter composition (>15%) allowed stress tolerance taxa, such as A. tepida, to flourish.
Foraminifera Species-Environment Relationship
The CCA results suggest that the distribution of benthic foraminifera within the Malacca Strait varies with water depth (Figure 5). The cluster analysis produced three groups of foraminifera assemblages (Figure 4), which represented the transition from shallow to deep water environments (Figure 5). The Group A assemblage represented samples obtained from locations with varying magnitudes of environmental gradients; agglutinated foraminifera were also present in these samples. This group can be further divided into subgroups A1 and A2. The foraminifera assemblages of A1, which correlated with deeper water and sandy substrates, showed a more diverse species distribution (average Fisher’s alpha = 6.43; Table 2). The abundance of agglutinated foraminifera was also higher in A1 than in other groups. For example, Textularia spp. (13–24%) and A. agglutinans (4–9%) showed relatively higher abundances at water depths of 55–71 m (stations W29–31). In contrast, the relative abundance of agglutinated foraminifera was lower in Group A2 and was correlated with higher percentages of mud. Within Group A2 assemblages, A. pulchella and E. advenum were more abundant in the muddy substrate. In addition, E. advenum only showed >15% relative abundance at stations W38 and W39, which belong to the Group A2 assemblages. Similar species have been reported in the muddy substrates of the Andaman Sea at water depths of 4–20 m (Gandhi et al., 2016). This explains the maximum abundance of this species at similar water depths within the Malacca Strait.
Group B was characterised by open marine foraminiferal assemblages. Most of the stations belonged to this group and had water depths of >40 m. Although A. pulchella was the dominant species in Group B, its relative abundance was much lower than that of the other cluster groups. Instead, Bolivina spp. had a higher relative abundance in Group B assemblages, and the maximum abundance of B. glutinata (38%) was observed at station W42. Bolivina spp. is opportunistic taxa that may tolerate low oxygen conditions and prefer substrates with higher organic matter content (Murray, 2006; Eichler et al., 2012). The Group B assemblages were associated with muddy substrates, with an average sediment organic matter content of 6%. Group C represented foraminifera assemblages from shallow water, with a much higher sediment organic matter (average 11.5%) content than other cluster assemblages. Similar to previous reports in organic-rich sediments, Ammonia spp. had higher relative abundances in Group C (Melis and Violanti, 2006; Minhat et al., 2014, 2020; Martins et al., 2015). In this study, A. tepida and A. convexa together represented >80% of the total foraminiferal assemblage at stations W36 and W37, which had organic matter contents of >15%. In addition, the much higher organic matter content in Group C may be the reason for the absence of agglutinated species, except for Textularia spp. Ammobaculites exiguus, and Trochammina sp. 1.
This study documented the distribution and ecological preferences of foraminifera species along the busy Malacca Strait. Based on our findings, species such as A. pulchella were found to be useful for paleo-salinity interpretations. Moreover, understanding the distribution of H. praecincta and D. bertheloti and their correlation with water depth can help to determine past depositional environments. The Malacca Strait is one of the busiest shipping regions in the world, as it connects several major shipping ports in East and West Asia (Cheng et al., 2019). As a result, it is affected by land-based pollution from littoral states and marine-based pollution from maritime activities (Yasin et al., 2019). Malaysia is a dominant littoral state in the Malacca Strait and therefore plays a major role in monitoring and governing marine health in the region. The benthic foraminifera data reported in this study are thus highly useful for monitoring the marine conditions in the region. Despite the high volume of terrestrial discharge from various rivers to the Malacca Strait, this study reported predominantly sandy substrates in most stations, similar to those reported by Keller and Richards (1967). In addition, we found that stations located closer to the coastlines experienced hyposaline conditions and organic matter enrichment. Similar to other shallow water environments around peninsular Malaysia (Minhat et al., 2016; Martin et al., 2018; Suriadi et al., 2019), the foraminifera assemblages in the Malacca Strait are dominated by calcareous hyaline groups. The extremely low relative abundance or absence of porcelaneous groups could be attributed to the low concentration of calcium carbonate along the strait (Keller and Richards, 1967).
Conclusion
The benthic foraminifera along the Malacca Strait were dominated by calcareous hyaline species with extremely low numbers of porcelaneous species. Asterorotalia pulchella was the most common and dominant species in this study because of the relatively low salinity along the strait. According to the CCA and cluster analysis, foraminifera assemblages correspond to the transition from shallow coastal water to deeper marine environments. Depth was, therefore, the major factor influencing foraminifera species along the Malacca Strait.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author Contributions
FM has proposed this study, funded the laboratory analysis, and contributed in major revision of the manuscript including statistical interpretation and writing the discussion. SG has contributed in critical revision of the manuscript with input on language and the flow of the manuscript. NA, NH, OM, and SS have involved in field samples collection, laboratory analysis of sediment foraminifera and organic matter content. LL, MAk, and MAb have contributed in the revision of the manuscript and research funding for field works. All authors contributed to the article and approved the submitted version.
Funding
This project was supported financially by the Fundamental Research Grant Scheme under the Ministry of Higher Education (FRGS/1/2018/STG09/UMT/03/01).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We would like to acknowledge the field and lab assistance provided by the entire team involved in the UMT Scientific Expedition Voyage LIMA’19. Special thanks to all researchers involved in TAPE-RG (Vot. No. 55186) project.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2021.647531/full#supplementary-material
References
Amiruddin, A. M., Ibrahim, Z. Z., and Syazwan, A. I. (2011). Water mass characteristics in the strait of Malacca using ocean data view. Res. J. Environ. Sci. 5, 49–58. doi: 10.3923/rjes.2011.49.58
Azmi, N., Minhat, F. I., Hasan, S. S., Rahman Abdul Manaf, O. A., Abdul A’ziz, A. N., Wan Saelan, W. N., et al. (2020). Distribution of benthic foraminifera off Kelantan, peninsular Malaysia, South China Sea. J. Foraminifer. Res. 50, 89–96. doi: 10.2113/gsjfr.50.1.89
Benito, X., Trobajo, R., Cearreta, A., and Ibáñez, C. (2016). Benthic foraminifera as indicators of habitat in a Mediterranean delta: implications for ecological and palaeoenvironmental studies. Estuar. Coast. Shelf Sci. 180, 97–113. doi: 10.1016/j.ecss.2016.06.001
Bouchet, V. M., Goberville, E., and Frontalini, F. (2018). Benthic foraminifera to assess ecological quality statuses in Italian transitional waters. Ecol. Indic. 84, 130–139. doi: 10.1016/j.ecolind.2017.07.055
Cheng, L., Yan, Z., Xiao, Y., Chen, Y., Zhang, F., and Li, M. (2019). Using big data to track marine oil transportation along the 21st-century maritime silk road. SCIENCE CHINA Technol. Sci. 62, 677–686. doi: 10.1007/s11431-018-9335-1
Chua, T., Gorre, I. R., Ross, S. A., Bernad, S. R., Gervacio, B., and Ebarvia, M. C. (2000). The Malacca Straits. Mar. Pollut. Bull. 41, 160–178.
Culver, S. J., Mallinson, D. J., Corbett, D. R., Leorri, E., Rouf, A. A., Mohd Shazili, A. N., et al. (2012). Distribution of foraminifera in the Setiu estuary and lagoon, Terengganu, Malaysia. J. Foraminifer. Res. 42, 109–133. doi: 10.2113/gsjfr.42.2.109
Dijkstra, N., Junttila, J., Skirbekk, K., Carroll, J., Husum, K., and Hald, M. (2017). Benthic foraminifera as bio-indicators of chemical and physical stressors in Hammerfest harbor (northern Norway). Mar. Pollut. Bull. 114, 384–396. doi: 10.1016/j.marpolbul.2016.09.053
Eichler, P. P., Eichler, B. B., Gupta, B. S., and Rodrigues, A. R. (2012). Foraminifera as indicators of marine pollutant contamination on the inner continental shelf of southern Brazil. Mar. Pollut. Bull. 64, 22–30. doi: 10.1016/j.marpolbul.2011.10.032
Evers, H., and Gerke, S. (2006). The Strategic Importance of the Straits of Malacca. ZEF Working Paper Series No. 17.
Gandhi, S. M., Rao, R. N., Raja, M., and Suresh, N. (2016). Benthic foraminiferal distribution and its ecological studies in shallow shelf setting off Colachel, Kanyakumari District, Tamil Nadu. Indian J. Mar. Sci. 45, 742–748.
Hallock, P., Lidz, B. H., Cockey-Burkhard, E. M., and Donnelly, K. B. (2003). Foraminifera as bioindicators in coral reef assessment and monitoring: the FORAM index. Environ. Monit. Assess. 81, 221–238. doi: 10.1023/A:1021337310386
Heiri, O., Lotter, A. F., and Lemcke, G. (2001). Loss on ignition as a method for estimating organic and carbonate content in sediments: reproducibility and comparability of results. J. Paleolimnol. 25, 101–110. doi: 10.1023/A:1008119611481
Hii, Y. S., Law, A. T., Shazili, N. A. M., Rashid, M. A., Lokman, H. M., Yusoff, F. M., et al. (2006). The straits of Malacca: hydrological parameters, biochemical oxygen demand and total suspended solids. J. Sustain. Sci. Manag. 1, 1–14.
Keller, G. H., and Richards, A. F. (1967). Sediments of the Malacca strait, Southeast Asia. J. Sediment. Res. 37, 102–127.
Kemp, A. C., Cahill, N., Engelhart, S. E., Hawkes, A. D., and Wang, K. (2018). Revising estimates of spatially variable subsidence during the AD 1700 Cascadia earthquake using a Bayesian foraminiferal transfer function revising estimates of spatially variable subsidence. Bull. Seismol. Soc. Am. 108, 654–673. doi: 10.1785/0120170269
Loeblich, A. R., and Tappan, H. (1988). Foraminiferal genera and their classification. New York: Van Nostrand Reinhold, 715.
Loeblich, A. R., and Tappan, H. (1994). Foraminifera of the Sahul shelf and Timor Sea. Vol. 31. USA: Cushman Foundation for Foraminiferal Research, Special Publication, 661.
Martin, S. Q., Culver, S. J., Leorri, E., Mallinson, D. J., Buzas, M. A., Hayek, L. A., et al. (2018). Distribution and taxonomy of modern benthic foraminifera of the Western Sunda shelf (South China Sea) off peninsular Malaysia. J. Foraminifer. Res. 48, 388–389. doi: 10.2113/gsjfr.48.4.388
Martins, M. V. A., Silva, F., Laut, L. L. M., Frontalini, F., Clemente, I. M. M. M., Miranda, P., et al. (2015). Response of benthic foraminifera to organic matter quantity and quality and bioavailable concentrations of metals in Aveiro lagoon (Portugal). PLoS One 10:e0118077. doi: 10.1371/journal.pone.0118077
Melis, R., and Violanti, D. (2006). Foraminiferal biodiversity and Holocene evolution of the Phetchaburi coastal area (Thailand gulf). Mar. Micropaleontol. 61, 94–115. doi: 10.1016/j.marmicro.2006.05.006
Mello, J. F., and Buzas, M. A. (1968). An application of cluster analysis as a method of determining biofacies. J. Paleontol. 42, 747–758.
Minhat, F. I., Satyanarayana, B., Husain, M. L., and Rajan, V. V. (2016). Modern benthic Foraminifera in subtidal waters of Johor: implications for Holocene sea-level change on the east coast of peninsular Malaysia. J. Foraminiferal Res. 46, 347–357. doi: 10.2113/gsjfr.46.4.347
Minhat, F. I., Shaari, H., Razak, N. S. A., Satyanarayana, B., Saelan, W. N. W., Yusoff, N. M., et al. (2020). Evaluating performance of foraminifera stress index as tropical-water monitoring tool in strait of Malacca. Ecol. Indic. 111:106032. doi: 10.1016/j.ecolind.2019.106032
Minhat, F. I., Yahya, K., Talib, A., and Ahmad, O. (2014). Benthic foraminiferal distributions as bioindicators in coastal waters of Penang National Park, Malaysia. J. Foraminifer. Res. 44, 143–150. doi: 10.2113/gsjfr.44.2.143
Murray, J. W. (2006). Ecology and application of benthic foraminifera. New York: Cambridge University Press, 422.
Nagendra, R., and Reddy, A. N. (2019). Benthic foraminifera response to ecosystem pollution in the Uppanar estuary, Tamil Nadu coast, India. J. Geol. Soc. India 93, 555–566. doi: 10.1007/s12594-019-1217-6
Nurruhwati, I., Ardiansyah, F., Yuliadi, L. P. S., and Partasasmita, R. (2020). Benthic foraminifera as ecological indicators in the Tunda Island waters, Serang District, Banten Province, Indonesia. Biodiversitas Journal of Biological Diversity 21, 3142–3148. doi: 10.13057/biodiv/d210735
Panchang, R., and Nigam, R. (2012). High resolution climatic records of the past ~489 years from Central Asia as derived from benthic foraminiferal species, Asterorotalia trispinosa. Mar. Geol. 307, 88–104. doi: 10.1016/j.margeo.2012.01.006
Prazeres, M., Martínez-Colón, M., and Hallock, P. (2020). Foraminifera as bioindicators of water quality: the FoRAM index revisited. Environ. Pollut. 257:113612. doi: 10.1016/j.envpol.2019.113612
Rao, N. R., Jayaprakash, M., and Velmurugan, P. M. (2013). The ecology of Asterorotalia trispinosa—new insights from Muthupet lagoon, southeast coast of India. J. Foraminifer. Res. 43, 14–20. doi: 10.2113/gsjfr.43.1.14
Schönfeld, J., Alve, E., Geslin, E., Jorissen, F., Korsun, S., and Spezzaferri, S. (2012). The FOBIMO (foraminiferal bio-monitoring) initiative—towards a standardised protocol for soft-bottom benthic foraminiferal monitoring studies. Mar. Micropaleontol. 94, 1–13. doi: 10.1016/j.marmicro.2012.06.001
Scott, D. B., and Medioli, F. S. (1980). Quantitative studies of marsh foraminifera distribution in Nova Scotia: implications for sea-level studies. J. Foraminifer. Res. 17, 1–58.
Sousa, S. H. M., Yamashita, C., Semensatto, D. L., Santarosa, A. C. A., Iwai, F. S., Omachi, C. Y., et al. (2020). Opportunities and challenges in incorporating benthic foraminifera in marine and coastal environmental biomonitoring of soft sediments: from science to regulation and practice. J. Sediment. Environ. 5, 257–265. doi: 10.1007/s43217-020-00011-w
Sreenivasulu, G., Praseetha, B. S., Daud, N. R., Varghese, T. I., Prakash, T. N., and Jayaraju, N. (2019). Benthic foraminifera as potential ecological proxies for environmental monitoring in coastal regions: a study on the Beypore estuary, southwest coast of India. Mar. Pollut. Bull. 138, 341–351. doi: 10.1016/j.marpolbul.2018.11.058
Suriadi, R., Shaari, H., Culver, S. J., Husain, M. L., Vijayan, V. R., Parham, P. R., et al. (2019). Inner shelf benthic foraminifera of the South China Sea, east coast peninsular Malaysia. J. Foraminifer. Res. 49, 11–28. doi: 10.2113/gsjfr.49.1.11
Szarek, R. (2001). Biodiversity and biogeography of recent benthic foraminiferal assem- blages in the south-western South China Sea (Sunda Shelf). PhD dissertation. Kiel: Christian-Albrechts Universität, Kiel.
Yasin, Z., Hwai, A. T. S., Abdullah, A. L., and Razalli, N. (2019). The ocean legacy of Melaka and sustainability in the straits of Malacca. J. Ocean Cult. 2, 58–73. doi: 10.33522/joc.2019.2.58
Keywords: Asterorotalia, organic matter, monitoring, water depth, LIMA scientific expedition
Citation: Minhat FI, Ghandhi SM, Ahzan NSM, Haq NA, Manaf OARA, Sabohi SM, Lee LH, Akhir MF and Abdullah MM (2021) The Occurrence and Distribution of Benthic Foraminifera in Tropical Waters Along the Strait of Malacca. Front. Mar. Sci. 8:647531. doi: 10.3389/fmars.2021.647531
Edited by:
Rajeev Saraswat, Council of Scientific and Industrial Research (CSIR), IndiaReviewed by:
Ajoy Kumar Bhaumik, Indian Institute of Technology Dhanbad, IndiaArun Deo Singh, Banaras Hindu University, India
Copyright © 2021 Minhat, Ghandhi, Ahzan, Haq, Manaf, Sabohi, Lee, Akhir and Abdullah. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fatin Izzati Minhat, fatinminhat@umt.edy.my; fatinminhat@umt.edu.my
 Fatin Izzati Minhat
Fatin Izzati Minhat Suresh M. Ghandhi
Suresh M. Ghandhi Nurul Syahirah Mohd Ahzan1
Nurul Syahirah Mohd Ahzan1