– 21 March 2011 Fukushima Nuclear Accident Update by IAEA [conversion factor: 1 pCi = 0.037 Bq, therefore 1 Bq = 27 pCi]. On 21 March 2011, World Nuclear News reported: “In the town of Kawamata, three milk samples showed above 300 becquerels per kilogram [1 kg of milk ~ 1 litre] in iodine.”
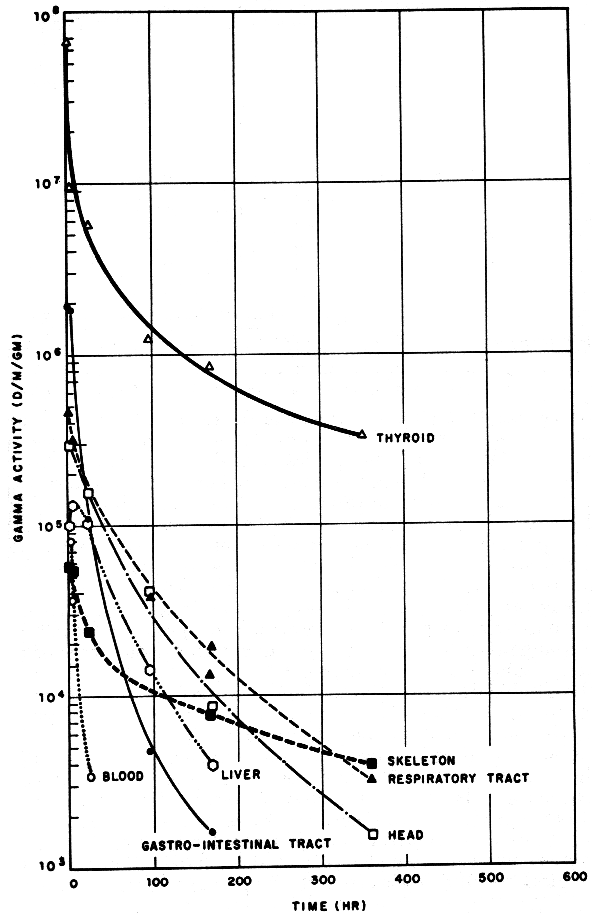
Above: in 1962, Salt Lake City in Utah was downwind from Nevada test site nuclear explosions Sedan (6 July, 104 kilotons, optimal depth for cratering, 12,000 ft high cloud), and the near-surface bursts Johnny Boy (11 July, 0.5 kilotons, 11,000 ft high cloud) and Small Boy (14 July, 1.65 kilotons, 15,000 ft high cloud).
Cows eat iodine-131 that lands on grass, and 3% of the ingested iodine is passed on to their milk. When milk is consumed, 25% of its iodine enters your thyroid gland. A single ingestion of 74,000 pCi (2,700 Bq) of I-131 by a child with a small (2 gram) thyroid gives a thyroid dose of 1 cGy or 1 rad. The same 1 cGy dose is delivered to the 2 gram child’s thyroid by consuming milk from an explosion (reactor or bomb) whose iodine-131 content peaks at 5,600 pCi/litre or 210 Bq/litre (source: UCRL-7716). As the graph above shows, the peak iodine-131 content of Salt Lake City milk was about 2,000 pCi/litre (74 Bq/litre) on 25 July 1962. Cresson H. Kearny pointed out that the maximum measured radioactive contamination of milk in the United States by iodine-131 from the 1986 Chernobyl nuclear disaster was just 560 pCi/liter of milk (produced by cows grazing on pasture in Washington), compared to 900 pCi/litre of iodine-131 in milk at Oak Ridge, Tennessee, after the 300-kiloton Chinese nuclear test explosion of December 28, 1968. With its 8 days half-life, the iodine-131 doesn’t last very long. Countermeasures are discussed below, after reactor safety facts.
Above: Fukushima Daiichi reactor designs from 1967-71, showing the deliberately frangible roof which blew off just as designed in the hydrogen gas explosions at Units 1 (12 March) and 3 (14 March), preventing any damage to the reactor cores after gas venting. The two-hour fire at the spent fuel storage pond beside Unit 4 on 15 March was not caused by the nuclear fuel, according to a statement by Japanese Prime Minister Naoto Kan’s spokesperson Noriyuki Shikata, and the storage pond was quickly and safely refilled with water, simply using a bucket carried under a Chinook helicopter:
Unit 1: Roof blown off by hydrogen gas explosion on 12 March
Unit 2: Torus under reactor exploded on 15 March
Unit 3: Roof blown off by hydrogen gas explosion on 14 March
Unit 4: Two-hour fire at the spent fuel storage pond on 15 March and another fire on 16 March
The faulty pressure relief valve and subsequent loss of coolant at Fukushima Daiichi Unit 2 led to a containment pressure increase to 700 kPa on 14 March, followed by the explosive rupture of the large torus steam-suppression chamber below the reactor pressure vessel on 15 March: “The pressure in the pool was seen to decrease from three atmospheres to one atmosphere after the noise, suggesting possible damage. Radiation levels on the edge of the plant compound briefly spiked at 8217 microsieverts per hour but later fell to about a third that. … Japanese authorities told the International Atomic Energy Agency (IAEA) that radiation levels at the plant site between units 3 and 4 reached a peak of some 400 millisieverts per hour … Later readings were 11.9 millisieverts per hour, followed six hours later by 0.6 millisieverts …”
This fast drop in radiation levels proves that the sources of the radiation are fast-decaying and dispersing gas and vapour (not a core explosion), and is not slow-decaying solid fuel particle deposits! The Unit 3 explosion on 14 March resulted in a peak site dose rate of 3.13 mSv/hour (~313 mR/hour) at 9:37AM (JST), falling rapidly to just 0.326 mSv/hour (~32.6 mR/hour) at 10:35 AM, less than an hour later, and to just 0.231 mSv/hour (~23.1 mR/hour) at 2:30PM, five hours after the initial peak. In addition, there was a fire on 15 March at the spent fuel cooling pond adjacent to Unit 4, but Japanese Prime Minister Naoto Kan’s spokesperson Noriyuki Shikata said that “we have found out the fuel is not causing the fire.”
Contrary to the BBC and other scare-mongering media, there has been no fuel meltdown, just a failure of the tops of the zirconium alloy “zircaloy” capsules at 1200 °C when the coolant water level fell below the tops of the fuel rods. This caused the zirconium to be oxidized by steam, releasing the hydrogen from the water, increasing the pressure and then exploding after being vented into the outer building as designed (diagram above). The uranium oxide ceramic fuel itself doesn’t melt below 2800 °C. The control rods were inserted to stop fission when the earthquake occurred on 11 March. Since then, the only heat source in the reactor has been the radioactive “decay heat” from fission products, not energy released by nuclear fission! The reactor cores have been under control and safely contained since 11 March.
Above: Japanese Prime Minister Naoto Kan’s spokesperson Noriyuki Shikata said that “we have found out the fuel is not causing the fire.” Helicopters were reportedly used to refil spent fuel storage pools, to cool the reactors and fight the fire (by dropping water on it from a safe height), to avoid danger to fire-fighters. The anti-nuclear biased BBC is having a field day, reporting the fire without mentioning that it is not a nuclear fuel fire, and presenting the event as if it is a repeat of the Chernobyl accident of 1986, when a dangerous RBMK reactor without a steel core protection went supercritical blew up after having the control rods completely removed while the safety system was turned off. In all the Japanese reactors affected, the control rods were fully inserted and nuclear fission stopped at the time of the earthquake on 11 March!
Safety of nuclear reactor cores from earthquakes and explosion blasts: the facts
On the 16 March BBC1 One Show, the BBC used the 1957 Windscale nuclear reactor fire in Cumbria to “explain” the dangers of the Japanese reactors. They omitted to mention that Windscale was an air-cooled burnable graphite moderated reactor with no steel containment vessel for the reactor core! The Japanese reactors used water as the coolant, which suppresses fire (unlike air). Also, the chief danger after the Windscale fire wasn’t fission products, but inhalation of polonium-210 which was being made in the reactor for the long-obsolete neutron initiators of old-fashioned nuclear bombs (modern nuclear bombs use miniature particle accelerator “zippers” as neutron sources). There is no polonium-210 in the Japanese reactors, which are used for energy production. Therefore, the situation is entirely different, so for failing with to point out these differences, the BBC was guilty of deliberate deception of its viewers. Modern nuclear reactors are entirely different.
Extensive research was done by the West (but not by the USSR) to ensure the safety of steel reactor cores vessels when a hydrogen gas or other explosion blast wave hits them. This is precisely why the Japanese reactor outer buildings had frangible roofs, designed to safely blow off in an explosion rather than to collapse and cause damage. The fear mongering American anti-nuclear politicians during the Cold War used to assume falsely that an explosion near a nuclear reactor would cause 100% of the core inventory to escape. That didn’t even happen in the explosion at Chernobyl (at full power and with the safety systems turned off), where the burning core was completely exposed to the atmosphere but only 3.2% of the plutonium escaped.
Dr Chester there evaluates a 1-GW nuclear reactor (which produces 3 kg of plutonium daily). The thick-walled reinforced concrete containment buildings resist peak horizontal ground shock (or earthquake) accelerations of 0.25g and much higher peak overpressures than ordinary buildings, typically 60 psi or 410 kPa for moderate damage (cracking but not total failure). The auxiliary diesel generators and control rooms are designed to withstand 25 psi or 170 kPa peak overpressure, but the steel pressure vessel containing the reactor core needs an overpressure impulse of at least 200 psi-seconds or 1.4 MPa-seconds to fracture it and cause a Chernobyl-type release. This is a really massive pressure impulse, which at a distance of just 0.7W2/3 metre from W kilotons of TNT equivalent nuclear explosion (see diagram below). A hydrogen gas explosion in the reactor building above the steel core vessel can’t produce such a powerful blast overpressure impulse, because it is not as powerful as a kiloton of TNT.
Even a direct hit by terrorists crashing a commercial aircraft into the outer building would not produce the pressures needed to rupture the steel pressure reactor vessel inside! As Dr Chester explained in 1988, modern nuclear reactors automatically shut themselves down in an accident when electric power is lost (gravity causes the control rods to fall into the core, shutting down fission) and they have efficient heat sinks with natural convection to remove decay heat without needing any external power, unlike the ancient 1967-71 designs in Japan. Dr Chester’s extensive published research includes:
“Civil Defense Implications of the Pressurized Water Reactor in a Thermonuclear Target Area,” Nuclear Applications and Technology, Vol. 9, 1970, pages 786-95
“Civil Defense Implications of a LMFBR in a Thermonuclear Target Area,” Nuclear Technology, Vol. 21, 1974, pages 190-200
“Civil Defense Implications of the U.S. Nuclear Power Industry During a Large Nuclear War in the Year 2000,” Nuclear Technology, Vol. 31, 1976, pages 326-38
Above: the overpressure-impulse from an air burst 1 kiloton or 1,000 tons of TNT equivalent nuclear explosion is only 10 kPa-sec or 1.4 psi-seconds at 100 metres and varies inversely with distance, 200 psi-seconds or 1.4 MPa-seconds of overpressure impulse (which Dr Conrad V. Chester of Oak Ridge National Laboratory calculates is needed to rupture the steel pressure vessel containing a nuclear reactor core) requires the distance between the steel reactor vessel and the 1,000 tons of TNT explosion to be just 0.7 metre (70 cm). This blast overpressure impulse can’t arise from a hydrogen gas explosion; there simply isn’t enough energy available! Extensive nuclear test data from the 1950s verify the resistance of steel to withstand nearby nuclear weapon detonations, with important implications for proving the survivability of nuclear reactor pressure vessels in any disaster, including a nuclear war. (Graph credit: John A. Northrop, Handbook of Nuclear Weapon Effects: Calculational Tools Abstracted from DSWA’s Effects Manual One (EM-1), Defense Special Weapons Agency, 1996.)
The ablation tests at the 23 kt Teapot-Met nuclear explosion in the Nevada on 15 April 1955 by J. E. Kester and R. B. Ferguson (Operation Teapot, Project 5.4, Evaluation of Fireball Lethality Using Basic Missile Structures, WT-1134, AD0340137), proved that at just 80 feet only the outer 0.4 inch of steel balls was ablated by the fireball. The steel vessels of nuclear reactors are at least 7.5 inches thick! The error in the popular myth that everything is totally vaporized in an explosion fireball is due to the fact that the cooling rate of the fireball is so great that there is literally not enough time for the heat to penetrate more than a thin surface layer before the temperature drops below the melting point of steel. Good heat conductors like steel are also protected by surface ablation.
At Chernobyl, 6.7 tons of radioactive debris blew off to great altitudes from a burning exposed reactor core, creating solid fallout particles like a nuclear surface burst explosion, which included the release of 75% of the reactor’s inventory of xenon-135 gas, 20% of the iodine-131 vapour, 15% of the tellurium-132, 12% of the caesium, 5.6% of the barium-140, 4% of the strontium, and 3.2% of Zr-95, plutonium and other refractory (high melting point) nuclides. In the Japanese reactor explosions, only a tiny quantity of the gas xenon and a trace of iodine vapour have escaped with the vented steam released from the steel reactor vessel. All of the debris seen in the explosions is non-radioactive frangible roof debris!



Above: we have long known all the facts about nuclear reactor explosion fallout because a Kiwi nuclear reactor was deliberately blown up at the Nevada test site by Los Alamos and the U.S. Naval Radiological Defense Laboratory on 12 January 1965, to determine the nature of reactor explosion fallout. It detonated with a nuclear explosion yield equivalent to 2.1 tons of TNT, and reached a maximum temperature of 4,250 K, which vaporised 5% of the reactor core fuel rods, of which 68% was dispersed as fallout with a specific activity of 1015 fissions/gram for refractory nuclides like Zr-95 (data: J. R. Lai and E. C. Freiling, Correlation of Radionuclide Fractionation in Debris from a Transient Nuclear Test, pages 337-51 of Radionuclides in the Environment, American Chemical Society, 1970). As that experiment’s projector officer, Dr Edward C. Freiling, observed in the 1970 book Radionuclides in the Environment, the physics of fallout fractionation in a nuclear reactor explosion is explained by Dr Carl F. Miller’s 1963 Fallout and Radiological Countermeasures calculations of fission product condensation. Most of the gases like xenon-135 and vapours with low boiling points like iodine-131 remain in the air and are quickly dispersed over large distances as a gas or on very small particles, unlike solids like plutonium which concentrate in large particles and don’t travel so far. In the Japanese reactors, only small amounts of gases were vented from the cores with steam, unlike Chernobyl. But the BBC doesn’t care:
”IT’S CHERNOBYL ALL OVER AGAIN!
“>> Tuesday, March 15, 2011“The BBC have gone nuclear over…erm, the nuclear problems at Fukushima. Today has been busy constructing an agenda that the Japanese Government ‘lies’ (according to Roger Harradin) and is ‘blasé’ (according to James Naughtie) about nuclear problems. Undoubtedly the crisis at Fukushima has gotten worse and that is fair comment but the BBC seems determined to extend this into some sort of general attack on nuclear energy. I have to say that one’s natural sympathy with the Japanese victims of the tsunami is now being eclipsed by anger about the BBC’s overt manipulation of the Nuclear power plant issue. Not reporting – editorialising and always following a clear agenda.
Posted by David Vance”
– http://biased-bbc.blogspot.com/2011/03/its-chernobyl-all-over-again.html
The Fukushima plant was only designed to withstand an 8.2 magnitude earthquake, but it withstood 9.0 earthquake and then complete inundation by a 25-foot tsunami. It is an old design which omits natural convection cooling for decay heat, which is a feature of modern reactor designs. So far over 10,000 people have been killed in the earthquake and tsunami, and only one person has been killed at the nuclear power station (the crane operator, when the crane collapsed). Compare that to the 290,000 annual deaths from carcinogenic soot pollution, or to the 73 deaths so far due to “safe” installed wind turbines (neglecting deaths during transport of parts, etc.), where 201 blade failures, 154 turbine fires, 108 structural failures, and 82 cases of environmental damage have occurred: “Pieces of blade are documented as travelling up to 1300 meters. In Germany, blade pieces have gone through the roofs and walls of nearby buildings.” (Source: Caithness Windfarm Information Forum, 31 December 2010. This data ignores all the terrifying long-term effects of wind warms, e.g. the risks to people who lose limbs or suffer brain damage when blades hit them on the head. Why take the risk of windfarms, when the casualty rates from a rival BBC-hated power source are so trivial by comparison?)
The Sendai earthquake moment magnitude of 9.0 is equivalent to a total energy of about 31,800 megatons of TNT, of which 1.5% or 477 megatons of TNT equivalent appeared as explosion-equivalent “surface waves” composed of ground shock motion and tsunami water waves, similar to the effects from ground-coupled shock energy in a surface burst explosion. The peak ground acceleration from surface waves is roughly 0.00014 E3/4/D2 g’s, where E is megatons of yield and D is distance from epicenter in km (source: formula is from Robert U. Ayres’s Hudson Institute report HI-518-RR, AD632279, 1965, Appendix E, page E-3).
Above: the explosion on 12 March 2011 of the outer concrete containment building of Japan’s Fukushima Dai-ichi nuclear reactor Number 1, the oldest of 11 reactors on the shoreline immediately exposed to the 9.0 moment magnitude earthquake and 7 metre tsunami which inundated emergency generators required for cooling the radioactive decay heat after shutdown. The reactor was designed in 1971 and is 170 miles northeast of Tokyo. Unlike the latest designs of nuclear reactors, this old reactor design does not allow natural convection cooling to dissipate the radioactive decay heat after shutdown, but requires a powered pump to keep the coolant flowing.
Above: Tuesday 15 March 2011 update on effects from Japanese Fukushima Dai-ichi nuclear reactor explosions. Note that all of the explosions were caused by water overheating and being reduced by heat into hydrogen and oxygen (which was then vented for safety into the outer buildings or external pipes, where it exploded safely with no reactor core vessel damage, and minimal radioactive xenon-135 gas contamination). Note that the overheating was not caused by nuclear fission but was due to radioactive decay heat (which decays quickly with time) after 10 nuclear reactors were shutdown following the earthquake. This issue was unique to those old reactor designs, because the Japanese 1967-1971 design required externally-powered coolant circulation after shutdown, unlike modern reactors which can dissipate the decay heat energy by natural convective cooling without any need for externally powered coolant pumps that are vulnerable to tsunami inundation!
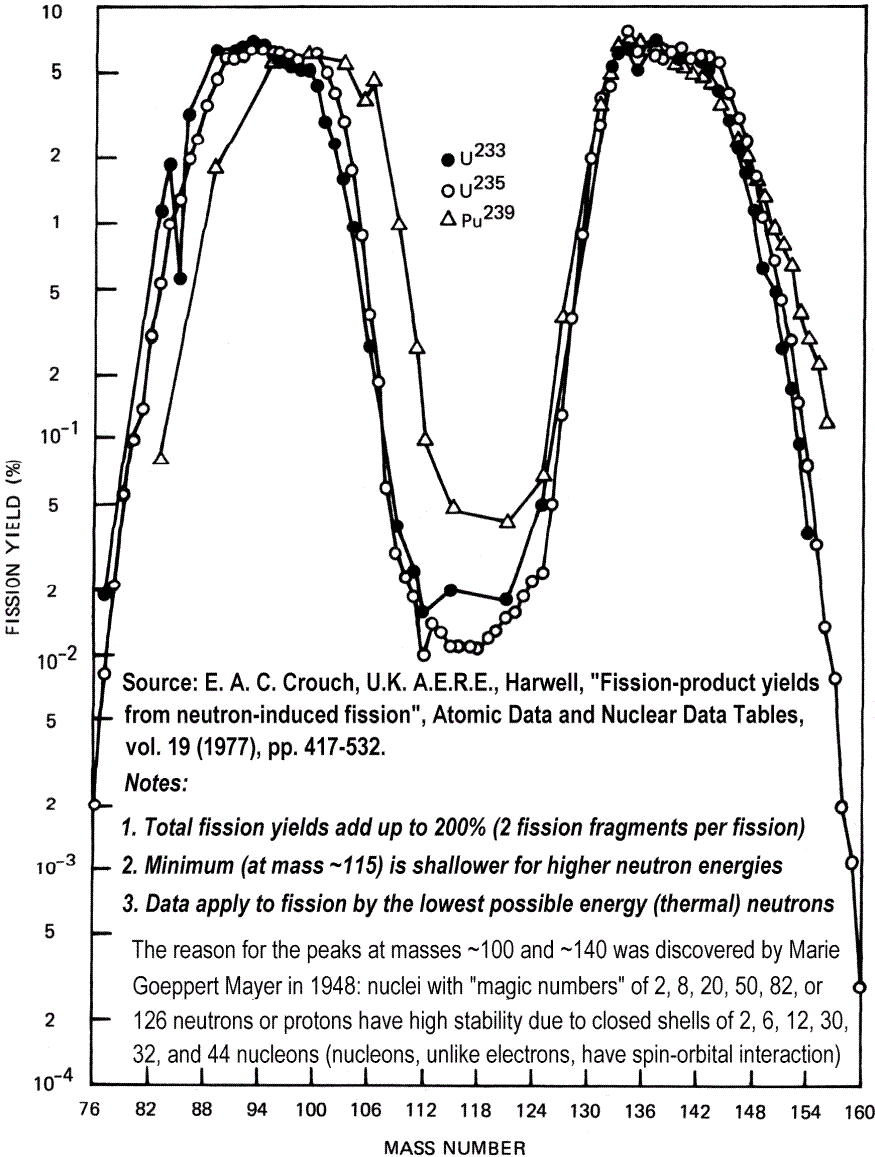
Fukushima Daiichi Unit 1 suffered a hydrogen gas explosion in the outer concrete building after venting of gas from the 15 cm thick stainless steel-protected reactor core vessel on 12 March, and Unit 3 underwent a similar hydrogen gas explosion on 14 March. Both explosions blew the roofs of the buildings, but the blasts did not damage the steel reactor core vessels. When the hydrogen gas from the cores was vented, some radioactive vapours like iodine-135 and particularly its more volatile “permanent gas” decay product xenon-135, escaped into the outer building from overheated zirconium fuel capsules (but no refractory nuclides like plutonium, which are solids, not gases). Iodine-135 vapour has a half-life of just 7 hours, and the gas xenon-135 has a half-life of just 9.2 hours, so even neglecting the fall in concentration due to atmospheric dispersion, less than 3% remains 48 hours later. Iodine-131 is also emitted, but gives lower initial dose rates due to its greater distance from the high mass number peak in the M-shaped fission product abundance distribution curve, and lower specific activity. This is because the longer the half-life, the longer the time taken between decays of radioactive atoms, so the lower the dose rate.
Ryohei Shiomi, official of the Nuclear and Industrial Agency, Japan, stated: “Units 1 and 3 are at least somewhat stabilised for the time being. Unit 2 now requires all our effort and attention.” Japan has evacuated 200,000 people from a 20 km (12-mile) radius exclusion zone around Fukushima Daiichi, and has warned people up to 30 km (19-miles) away to stay indoors and to wear a wet cloth over the face to absorb iodine vapour if they venture outdoors. They have 230,000 units of stable potassium iodide tablets read for dispersion in the highly unlikely event that a significant iodine-131 release occurs, to block iodine-131 uptake by the thyroid gland and therefore prevent the thyroid cancers that were reported after the Chernobyl accident in 1986.
In depth
The decay heat in the steel pressure vessel converted the stagnant coolant water into high pressure steam and a mixture of hydrogen and oxygen gas, which had to be vented into the concrete containment building surrounding the 15 cm thick steel reactor core vessel. The hydrogen and steam mixture in the outer building then exploded, blowing the concrete roof off and allegedly venting a small quantity of gaseous fission products, mainly xenon-135, from damaged fuel rods (the zirconium casings of fuel rods melt at 1200 C), which had been released into the outer building along with the steam and hydrogen gas during the pressure venting. The strong steel reactor core remained intact.
After the Unit 1 explosion on 12 March, the radioactivity level inside the concrete containment building reached 100 microSieverts per hour or 10 mR/hour (1000 times natural background radioactivity). Outside the building, it reached 0.8 microSieverts per hour or 0.08 mR/hr (8 times background). Chief Cabinet Secretary Yukio Edano said radiation around the plant had fallen after the time of the blast, confirming that the steel fuel vessel is intact. Sea water is now being used to cool down the reactor core, as is the case at reactor number 3.
The episode proves the safety of nuclear power in the worst case scenario of an 9.0 moment magnitude earthquake followed by a massive tsunami which destroyed backup power for coolant circulation, was safe for 11 reactors and caused only a minor venting of short-lived volatile nuclides with a maximum radiation level outside the oldest reactor buildings of 8 times background, which rapidly decayed and dispersed. The volatile radioactive fission product gas xenon-135 has a 9.2 hours half life, so under 3% remains after 48 hours or 5.2 half lives, and in addition it is quickly dispersed and diluted to safe levels. The initial maximum dose rate outside the building of 8 times normal background would be down to just 0.2 times background just assuming xenon-135 decay, and neglecting the dispersion effect. This extreme proof test of the safety of nuclear power even under a an immense earthquake and tsunami is one piece of good news to come from the natural devastation scene in Japan. Lucky they didn’t have a wind farm in the devastated area, or the falling turbine blades would have caused a real additional danger, while solar cells would have been swept along as hazardous debris in the tsunami!
Above: it’s mainly gaseous xenon-135 with a half life of 9.2 hours that’s escaped, and less than 3% of that remains after 48 hours. Also, it mixes rapidly with the air and gets diluted, too. Notice that background is 0.01 mR/hour so the peak dose rate of 1000 times higher is 10 mR/hour. If this decays with 9.2 hours half life, the total dose with exponential decay is simply the initial peak dose rate times the mean life (for exponential decay the mean life is 1/ln 2 or 1.44 half lives). Hence total dose = 10 x 1.44 x 9.2 = 132 milliRoentgens. This is about the dose you get naturally over a year. No significant plutonium will have vented, because it’s a solid, not a gas. You only need to take potassium iodine tablets if the total predicted dose exceeds 20 Roentgens to the thyroid. The amount of iodine-131 released will be far less than the xenon-135 because it’s mass number is further from the peak on the M-shaped fission product abundance curve (scroll down for this fission product abundance curve), it has a higher boiling point than gases like xenon, and its longer half life (8 days) reduces its specific activity per atom. Other factors being similar, specific activity for a given number of radioactive atoms is inversely proportional to the half life. The longer the half life, the lower the dose rate because the same amount of radiation is given out more slowly. If it’s longer than a human life span, then you are saved all the radiation that is not released during your life! If they had wind turbines or solar cells in Japan with the same power output, they would have caused a serious hazard from flying panel debris, wind blades, etc., during the earthquake and tsunami.
1. Don’t drink fresh milk for a few weeks if the cattle are eating pasture grass contaminated with fresh fallout. (You can still use the milk to make cheese to be eaten after the 8-day half life of the iodine-131 has ensured its decay to insignificance.)
2. Or, continue using the milk so long as you can put the cattle indoors on winter feed until the iodine-131 (which has a mere 8 days half-life) has decayed.
3. A third option – which is not sensible unless the thyroid dose is expected to exceed 25 R – is to administer 130-milligram potassium iodide tablets to everyone daily who is drinking contaminated milk within a month of detonation; this blocks iodine-131 uptake by the thyroid by saturating it. But the evidence is that the risk of getting iodine-131 induced thyroid cancer from long-range fallout is so low that, in general, the low-risk of side effects from potassium iodide are similar or greater than those for radiation. The U.S. Federal Drugs Administration evaluated the risks of administering potassium iodide for thyroid blocking under emergency conditions:
‘FDA guidance states that risks of side effects, such as allergic reactions, from the short-term use of relatively low doses of potassium iodide for thyroid blocking in a radiation emergency, are outweighed by the risks of radioiodine-induced thyroid nodules or cancer, if the projected dose to the thyroid gland is 25 rems or greater.’
It’s worth adding that plutonium in soil is strongly discriminated against by land plants and animals. If you have 100 Bq/gram of plutonium measured in dried topsoil samples, the plutonium uptake in plants ranges from a maximum of 0.016 Bq/gram in dried broccoli (concentration factor 1.6 x 10-4) down to just 0.0029 Bq/gram in dried corn (concentration factor 2.9 x 10-5). So the plant discrimination against plutonium gives a protection factor of 6,300-34,000. In addition, when you eat plants containing plutonium, only 1 part in 10,000 is taken up from the gut and the rest is eliminated. So the combined plant and human discrimination against plutonium therefore means the plutonium concentration in your body (Bq/gram) is between 63,000,000 to 340,000,000 times less than that in the soil!
The soil is naturally radioactive with alpha emitters anyway: earth’s crust is composed of 4 parts per million uranium-238, and 12 parts per million thorium-232. Ingested uranium is more hazardous as a chemical heavy-metal poison to the kidneys than due to its radiation, since 2 micrograms uranium per gram of kidney is chemically toxic (the LD50). Americium-241 in household ionization smoke detectors (0.9 microcurie per smoke detector) emits 5.6 MeV alpha particles, compared to just 5.2 MeV alpha particles from plutonium-239. So household smoke detectors contain something emitting “deadlier” higher-energy alpha radiation than plutonium-239! In fact, it is very easy to stop alpha radiation, since it can’t penetrate unbroken skin. Inhaled particles are removed from the lungs unless they are just the right size (1-5 microns) to get into the alveoli. Even then, they must be insoluble if they are to remain there for any time, or else they will be dissolved and eliminated from the body naturally.
Plutonium can be concentrated in the marine ecosystem, but not by a large enough factor to overcome the dispersion in the oceans and the insolubility of plutonium, and in fish it concentrates in the inedible parts not the muscle. This was proved in 1973 by a major radiological survey of Eniwetok Atoll, where 43 nuclear weapons were tested in the atmosphere, with a total yield of 30 megatons of TNT equivalent. Measurements of plutonium in over 800 fish from Eniwetok Lagoon proved that a fish diet for 30 years will produce a human liver and bone radiation dose of just 0.1 mSv from plutonium, insignificant compared to over 1 mSv/year from natural radiation!
“In any event, milk collected during a period of high activity could be safely used for processed milk products and need not be thrown away. … An alternative but more expensive procedure would be to feed cows stored fodder until the iodine-131 activity in the pasturage declined to safe levels.”
What about caesium-137 (30 years half-life) and strontium-90 (29 years half life)?
As proved recently at Bikini Atoll, adding potassium fertiliser to lime rich soil (like coral sand, basically calcium carbonate) effectively blocks most of the uptake of caesium-137. Adding potassium chloride to the coral soil of Bikini Atoll, scene of 23 atmospheric nuclear weapons tests, totalling 77 megatons of TNT equivalent in the 1950s, reduced the Caesium-137 in coconuts by a factor of 20 from 3,700 Bq/kg to just 185 Bq/kg. Extensive detailed research on such brilliant “Fallout and Radiological Countermeasures” was instituted at the U.S. Naval Radiological Defense Laboratory in the 1950s by Dr Carl F. Miller, including simple, quick, cheap and highly efficient decontamination procedures for cities and agricultural areas!
In the 1986 Chernobyl nuclear accident, caesium-137 was the major long-lived contaminant but the concentration decreased rapidly with downwind distance, the deposition of Cs-137 being 250,000/D1.67 GBq/km2 at D kilometres downwind (for D beyond 10 km) (source: A. Aarkrog, “The radiological impact of Chernobyl compared with that from nuclear weapons fallout”, Journal of Environmental Radioactivity, vol. 6, 1988, pp. 151-62).
Ayres reports in table 1-7 on page 1-44 that 1 mCi of soluble Sr-90 deposited per square statute mile produced a final root-uptake equilibrium peak of 4.1 Becquerels (Bq) of Sr-90 per kg of human bone calcium, while for either green vegetables or root crops the same deposition produced 15 Bq/kg of human bone calcium, and for cereals it was 7.4 Bq/kg. The root uptake of Sr-90 from soil was reduced in areas with low soil calcium by simply adding lime to the soil; the calcium crowded out much of the Sr-90, which is already discriminated against by plants and animals. Deep ploughing put the contaminated to-soil below the average depth of the root crops cutting the Sr-90 uptake quickly, although the success of this approach depends upon the water table and the soil cohesion. Growing crops with low calcium content reduces the uptake of Sr-90 (potatoes contain only 1 mg of calcium per 10 calories). (Ayres reports his figures in “SU”, the old politically-incorrect 1953 RAND Corporation “sunshine unit” or “strontium unit”, defined as “1 micro-micro-Curie of Sr-90 per gram of bone calcium”: we have converted to Becquerels per kg for simplicity. 1 Curie = 1 Ci = 3.7 x 1010 Becquerels.)
The mainstream groupthink deception over radiation hormesis
Robin Clarke ignorantly asserts on page 9 of his 1975 book Notes for the Future: “the lethal ‘side-effects’ of radiation from a nuclear reactor are not so different from those of the bomb itself, except in scale.”
So let’s explore the history of censorship of the dependence of dose rate on the effects of radiation, called hormesis. The effects at Hiroshima and Rongelap were due to extremely high doses received at extremely high dose rates which prevented DNA repair enzymes to repair the double stand breaks as they occurred (which occurs at lower dose rates), while Chernobyl’s widespread effects were provably due to radiophobia – the simply false reporting of natural cancer and natural genetic effects as due to radiation, often “justified” by non-existent, inappropriate, or poorly-diagnosed “unexposed control groups”. If you diagnose 100% of the natural cancer in an irradiated group but only 50% of the cancer in a “control group”, then you will claim that the risk of cancer in the irradiated group is double that in the unexposed “control group”, when it’s simply a difference in diagnosis rates due to the radiophobia-induced hypochondria. The irradiated, scare-mongered group will be more likely to report any possible cancer symptoms than the unirradiated group.
Above: low dose rates of radiation stimulate growth in mice, evidence of radiation hormesis (from Dr T. D. Luckey, Radiation Hormesis Overview, lecture given at ICONE-7, Tokyo, April, 1999). We discussed the source of errors in the mainstream linear, no-threshold extrapolations from Hiroshima and Nagasaki data in previous posts: they apply to very high dose rates (initial nuclear radiation received over a period of seconds), and are extrapolated downwards using the linear law of genetic effects in non-mammalian, short-lived insects (Muller’s fruit flies), and also plants like maize. Insects and plants like maize don’t live for decades before reproduction, so they don’t acquire significant doses of natural background radiation, and they don’t need to evolve DNA repair enzymes (unlike mammals which produce relatively few offspring after a period measures in decades). So the present radiation dose standards are based on a false radiation effects model from insects and plants (dating back to anti-nuclear bias by Edward Lewis in 1957 Congressional Hearings on fallout, as documented in detail in previous posts), which must be revised to take account of mammalian DNA repair enzyme (e.g., protein P53) stimulation as a form of cancer prevention at low dose rates. This stimulation is akin to overcompensation by muscles to regular exercise: an increased rate of DNA breakage leads the body to devote more metabolism to DNA repair enzymes like protein P53, which overcompensates. You reduce the cancer risk by devoting additional energy to DNA repair enzymes than is normally used in that manner, an analogy to reducing a fire risk by spending more money on fire sprinkler systems or fire resistant materials, as Dr Jeffrey Moss explains in the video about hormesis below:
Above: hormesis is dose rate dependent, not just dose dependent! Radiation or chemical induced double strand DNA breaks occurring at a rate faster than they can be repaired by DNA repair enzymes in cell nuclei (such as protein P53) results in a net increase in cancer risk, while lower dose rates can stimulate the whole DNA repair enzyme system to repair breaks more efficiently than they do naturally. This is seen clearly in skin cancer, from high dose rates of ultraviolet radiation. See also the posts here and here.
Above: the loss of naivety in dose-response relationships. The optimum curve, effect probability = e-bA – e-cA, represents the stimulation of the DNA repair enzyme system by radiation dose rate A. At high dose rates, the DNA repair enzyme system is itself damaged by and unable to function efficiently, but at lower dose rates it is stimulated by radiation into working faster. However, historically the discovery of DNA repair enzymes only date from the 1970s, and data on non-linear radiation effects from earlier periods was ruthlessly censored (mainly by anti-nuclear fallout political propaganda and scare-mongering) in deference the simplest idea, the linear dose-effecs law, where effects are supposedly directly proportional to causes. However, you soon learn in most medicines that increasing the dose of a vitamin or other drug doesn’t actually improve the effect without limit. Either a saturation point is produced, beyond which subsequent doses are simply wasted, or – worse – an overdose produces smaller benefits than lower doses! E.g., if the overdose side-effects of a massive dose from aspirin kill most people by internal bleeding, then the overall beneficial effect of increasing the dose drops when the optimum dose rate is exceeded, instead of either increasing or remaining constant! Although the mathematical theory of natural exponents which produce the realistic dose-effects curves have been known for a long time (the constants are easy to fix from the linear law for very small doses, and from experimental data on large doses), there is an Orwellian “doublethink” or “crimestop” brainwashing system in place in groupthink science dogma hype, which prefers to endlessly promote false linear laws. To get the facts to “fit” such false laws, the data is fiddled by the simple process of natural selection: disregarding as “suspect” any data that doesn’t conform to the mainstream reigning science dogma and bias! Exactly the same mechanism led to a gradual evolution of experimental measurements of fundamental constants like the electronic charge: the first investigators made errors but were revered. Subsequent investigators were awed by the first investigators, and feared any data which diverged too far from it. So they deleted as “suspect” most of the correct data, being biased in favour of incorrect figures that confirmed the mainstream prejudice! Only in gradual steps, paper after paper, did the consensus shift towards the correct values.
– James Delingpole, How to be right, Headline, London, 2007, pages 113-4.
Above: Roddy Campbell’s post begins with the photo above of the mutant horse found near Chernobyl: “This mutant pony – pictured near Chernobyl – has 11 bodies, 11 heads and no fewer than 44 legs.” It continues:
– Roddy Campbell, “Nuclear power – some perspective”, guest post on James Delingpole’s Telegraph blog, 14 March 2011.
But 1% of 4,000 equals 40 deaths downwind (off-site) from Chernobyl. For iodine-131 (half life 8 days) there are simple antidotes like not drinking contaminated food and water, or taking potassium iodide or iodate tablets (130 mg per day). These flood the thyroid gland with stable iodine, preventing update of 99% of the iodine-131. The death figure then goes down to 1% of 40, i.e. no expected casualties.
“… it is important to note that, given the effects of a few seconds of irradiation at Hiroshima and Nagasaki in 1945, a threshold near 200 mSv may be expected for leukemia and some solid tumors. For a protracted lifetime natural exposure, a threshold may be set at a level of several thousand millisieverts for malignancies, of 10 grays for radium-226 in bones, and probably about 1.5-2.0 Gy for lung cancer after x-ray and gamma irradiation. The hormetic effects, such as a decreased cancer incidence at low doses and increased longevity, may be used as a guide for estimating practical thresholds and for setting standards. …
“The highest average thyroid doses in children (177 mGy) were accumulated in the Gomel region of Belarus. The highest incidence of thyroid cancer (17.9 cases per 100,000 children) occurred there in 1995, which means that the rate had increased by a factor of about 25 since 1987.
“This rate increase was probably a result of improved screening [not radiation!]. Even then, the incidence rate for occult thyroid cancers was still a thousand times lower than it was for occult thyroid cancers in nonexposed populations (in the US, for example, the rate is 13,000 per 100,000 persons, and in Finland it is 35,600 per 100,000 persons). Thus, given the prospect of improved diagnostics, there is an enormous potential for detecting yet more [fictitious] “excess” thyroid cancers. In a study in the US that was performed during the period of active screening in 1974-79, it was determined that the incidence rate of malignant and other thyroid nodules was greater by 21-fold than it had been in the pre-1974 period.”
The normal thyroid “nodule” incidence is 16% in Americans, and 35.6% in the more carefully screened Finland population. A large percentage of people have thyroids that don’t conform to the medical textbook. What happens after a nuclear accident is that people go looking for these nodules, feeling people’s throats, and detecting more of the natural incidence, then mis-reporting this rise in detection of natural thyroid gland “deformalities” as radiation-induced nodules. At Rongelap atoll, where people received a really massive thyroid dose of 2,100 R or 21 Gray from drinking water from an open cistern for two days before evacuation 115 miles downwind of the 15 megaton Bravo nuclear test on 1 March 1954, some really did get thyroid cancer (source: Dr Edward T. Lessard, et al., Thyroid Absorbed Dose for People at Rongelap, Utirik, and Sifo on March 1, 1954, BNL-5188). But the highest dose in kids thyroids after Chernobyl was only 177 mGy or 0.177 Gray, over a hundred times lower than the 18 Gray thyroid dose at Rongelap! It seems that all Chernobyl thyroid cancers are claimed to be natural cancers, under the threshold cancer dose, and due to screening!
The same occurred with genetic effects immediately after Hiroshima and Chernobyl. E.g., the BBC and newspapers had an episode after of Chernobyl where they visited clinics filled with special needs children downwind of Chernobyl, and tried to claim that these children were proof of the evil of nuclear power, regardless of the natural incidence. Some clinic directors cooperated, to get funding, which was needed (no problem there!). The problem was the big lie of obfuscating natural incidences of genetic effects, cancer, and thyroid “malformations” with radiation for deliberate anti-nuclear scaremongering.
Because the scientific community were unable to communicate such facts efficiently against pseudo-scientific propaganda, over 100,000 human lives were lost by abortions after Chernobyl: in 1995, environmentalist Michael Allaby stated on pages 191-7 of his book Facing the Future: the Case for Science (Bloomsbury, London):
“The clear aim of the anti-nuclear movement is to silence all opposition … theirs are now the only voices heard … In the Gomel district … which was one of the most heavily contaminated [after the Chernobyl nuclear disaster of 1986], the death rate per thousand newborn babies was 16.3 in 1985, 13.4 in 1986, and 13.1 in 1987; in Kiev region the figures … were, respectively, 15.5, 12.2, and 12.1.”
The International Atomic Energy Authority has reported that over 100,000 excess abortions were performed throughout Western Europe after the Chernobyl accident (reference: L. E. Ketchum, Lessons of Chernobyl: SNM members try to decontaminate world threatened by fallout, Part I [Newsline], J. Nucl. Med., vol. 28, 1987, pp. 413-22). This is the danger from lying. The newspapers and media generally have a vested interest in hyping anti-nuclear lies to make a big “splash” that sells newspapers.
It’s all phoney, extrapolating linearly down from effects at massive doses and massive dose rates despite non-linear response rates, or falsely claiming that improved diagnosis rates correlate to effects from radiation. The whole reason why nuclear power is currently expensive is political fear-mongering over lying radiation “risks”, proven by even more obvious groupthink fakery than the photograph of the “44 legged mutant horse” from Chernobyl which Delingpole gives. This pushes up the costs of reprocessing spent fuel, because it has to be done in laboratory-type glove boxes, with staff restricted to tiny doses. E=mc2 tells you that 1 kg converted into energy gives 9 x 1016 Joules of energy. Fission converts 0.1% of uranium-235 into energy, so fissioning 1 kg of uranium-235 produces 9 x 1013 Joules of energy.
Done efficiently with cheap reprocessing and with the surplus neutrons being captured in cheap and abundant uranium-238 to form plutonium-239 (or captured in cheap and abundant thorium-232 to form uranium-233), nuclear power would be the cheapest power on earth. The whole problem is psychological “groupthink” against small doses of radiation, despite the fact we get doses all the time.
The reason why you can’t extract dinosaur DNA from a fossil mosquito in amber 65 million years old is that the DNA has been totally broken down by the natural background nuclear radiation dose exceeding 6 million centigray over that period. DNA in living cells has received the same dose while being passed on during all those generations, but because of DNA repair enzymes in mammals (as distinct from natural selection in simple insects like Muller’s notoriously misleading X-rayed fruitfly mutations), the damage has been repaired.
Why there is media ignorance and prejudice on the safety of nuclear radiation and energy
The problems facing public perception of nuclear power begin with the dismal fact that nuclear physics is a notoriously obfuscated empirical science! For example, the theory of quantum chromodynamics is extremely difficult to solve even for relatively simple interactions due to the divergence of the path integral’s perturbative expansion caused by the large running coupling for the strong nuclear interaction. Successively more complex terms in the quantum chromodynamic expansion correspond to Feynman diagrams with ever increasing contributions to the path integral, so they cannot be ignored as is done in quantum electrodynamics (where successive terms have smaller contributions, due to the relatively small value of electromagnetic alpha). The Standard Model of particle physics is too complicated for nuclear physics where you have a large number of nucleons. Therefore, instead of using one theory to calculate everything in nuclear physics, it is built on various empirical models of the nucleus: a liquid droplet for fission, but the gamma ray line spectra from nuclear radioactivity indicates a definite shell structure, analogous to the shells of electrons in atomic physics! The inability to picture the nucleus clearly has not helped the public at large to understand nuclear physics. Mathematics has turned the subject into an unpopular occult priesthood, while bomb plutonium production for deterrence, using nuclear reactors, introduces secrecy and militarism.
There are different models of the nucleus used for different purposes, reminding you of the original confused response of many physicists to Louis de Broglie’s theory of wave-particle duality (de Broglie, and his friend David Bohm, believed in some kind of space-time fabric – don’t call it aether – which oscillates like a wave as a particle travels through it). Einstein’s E = mc2 doesn’t explain nuclear energy, since you can use the same formula for non-nuclear energy, e.g. the “potential energy” of electromagnetic fields (the “binding energy” for chemical reactions) in an ordinary battery is equivalent to a tiny mass increase. When a chemical battery is discharged, the energy it loses causes a tiny fall in mass, exactly as predicted by Einstein’s mass-energy equivalence. Therefore, Einstein’s E = mc2 is not unique to nuclear energy, and is provably an obfuscation when applied to nuclear reactions but not to chemical reactions! Most people who listen to the “Einstein equation explanation” on nuclear energy know they don’t gain any understanding from it, because it explains nothing in a useful way, even ignoring the fact that the equation also applies to chemical energy. So they feel insulted, patronised, and annoyed by this self-indulgent simplistic obfuscation from physicists.
Above: the misleading curve of nuclear binding energy (credit: Dr David Langford, who points out that although beryllium 8 “should” be stable, it “in practice flies apart to give two helium nuclei”). The “binding energy per nucleon”, peaking at 8.7 MeV/nucleon for nickel-60, is the mean amount of energy needed to free a nucleon (neutron or proton) from its nucleus. Since this is always above 1 MeV on the graph above, you would think that no particle with less than 1 MeV could ever possibly cause a nucleus to break up! However, the average binding energy can be very misleading, since the nucleons in the outer shells of the nucleus are less strongly held, and the fields holding them aren’t classical continuously-operating fields, but are particle-mediated, fluctuating fields. Therefore, some nuclei can emit neutrons spontaneously despite the average values of nuclear binding energy shown! In addition, low energy neutrons (below 1 MeV energy) can for odd-mass number heavy elements 233, 235, 237, 239, and 241, but not even masses 232, 234, 236, or 238, induce nuclear reactions like fission. Odd-mass numbers imply incomplete nuclear subshells and therefore higher nuclear instability and reactivity, just as occurs with chemical element atomic (not mass numbers). Even numbers imply fully paired-up particles. The fact that the atomic (proton) number is important for chemistry, not the mass (nucleon) number (vice-versa for nuclear physics), shows that the strong nuclear force determining the nuclear shell structure does not depend on electric charge. This led Heisenberg to argue that each nucleon, whether electrically positive or neutral, has similar nuclear “isospin” charge.
The nucleus is 10,000 times smaller in radius than the entire atom, but contains nucleons having either positive or neutral electric charges, all nearby in that tiny volume. Since the electromagnetic force is an inverse-square law force, in the nucleus it stronger than for electrons by a factor of about (10,000)2 = 100,000,000. Therefore, the electromagnetic forces between protons in the nucleus are on the order of 100 million times stronger than the electromagnetic forces of chemistry which bind orbiting electrons to nuclei. Because of this immense electrostatic repulsion between protons, the nucleus would explode if it were not for the “strong nuclear” attractive force between protons and neutrons, which is due to the exchange of “virtual pions”. The virtual pions are off-shell particles which are created by pair production, which occurs in strong electric fields (exceeding Schwinger’s threshold of 1.3 x 1018 volts/metre, for steady electric fields).
This exchange of virtual pions between nucleons, cause the attraction that prevents nuclei from exploding, and when you fire a neutron into uranium-235 it upsets the balance between electromagnetic repulsion and virtual pion-mediated attraction, and causes the nucleus to fission. The electromagnetic repulsion between protons is continually trying to explode the nucleus, and being thwarted by the nuclear strong force, mediated at long distances by virtual pion exchange. Therefore, nuclear explosions are really caused by electromagnetic repulsive energy between protons overcoming nuclear attractive binding energy forces! The reason why the electromagnetic repulsive force causes nuclei to break up and release so much more energy than is given off in chemical explosions is simply that the nucleus is 10,000 times smaller in radius than the atom, but contains a similar amount of electric charge (the number of protons in the nucleus is equal to the number of orbital electrons, unless the atom is charged), so by Coulomb’s inverse-square law the nuclear electromagnetic repulsive forces are (10,000)2 = 100,000,000 stronger than those involved between orbital electrons and nuclei.
What’s interesting next is the M-shaped distribution curve for the fission product abundance. The liquid drop predicts – wrongly – that two approximately equal droplets will be most likely, but in reality the most likely combination is for one fission product to have a mass considerably larger than the other. This is due to the relative stability of different combinations of nuclear shell-structures:
The unnecessary deaths due to the political radiation hormesis cover up by anti-nuclear lobby
The whole nuclear industry is in limbo on this, they’re mainly conservative and believe the best way to resolve any crisis is to do nothing, and say nothing. The anti-nuclear lobby uses falsified statistics that are complete lies, but they gain ground because hardly anybody defends the facts. One typical ploy is the lying claim that there is no human proof of hormesis or thresholds (ignoring the radium painters and Hiroshima), and that animal data is inadmissible.
More recently, there was a fine piece of mice research by Kazuo Sakai, Iwasaki Kazuo, Toshiyasu Iwasaki, Yuko Hoshi, Takaharu Nomura, Takeshi Oda, Kazuko Fujita, Takeshi Yamada, and Hiroshi Tanooka, International Congress Series (2002) 1236 (Radiation and Homoeostasis): 487–490. They found that a dose rate of 1 mGy/hour (100 mR/hour or 10,000 times natural radiation background) stops cancer, and a further paper by Sakai and collaborators in 2006 gives statistically significant evidence that 0.7 mGy/hour extended the life expectancy of mice by 15% (Sakai has nice colour photos showing the slower aging of the irradiated mice, shown above).
“Today we have a population of 2,383 [radium dial painter] cases for whom we have reliable body content measurements. . . . All 64 bone sarcoma [cancer] cases occurred in the 264 cases with more than 10 Gy [1,000 rads], while no sarcomas appeared in the 2,119 radium cases with less than 10 Gy.”
– Dr Robert Rowland, Director of the Center for Human Radiobiology, Bone Sarcoma in Humans Induced by Radium: A Threshold Response?, Proceedings of the 27th Annual Meeting, European Society for Radiation Biology, Radioprotection colloquies, Vol. 32CI (1997), pp. 331-8.
The higher the dose rate the lower the threshold dose for effects, just as with aspirin. The radium dial painters had their bones irradiated by deposited radium over typically 30 years. Rowland could measure the radium in the bones after they died to determine the dose rate accurately, so this is reliable data (he even exhumed skeletons to get data). His funding was cut off when it became clear that there was a massive threshold dose needed for bone cancer if the dose was spread out. For Hiroshima nuclear bomb data, the dose rate was much higher so threshold dose for cancer was only a few cGy.
There is plenty of data proving that it’s the dose rate and not the old 1950s dose that really matters, because DNA repair enzymes like protein P53 are overloaded at high dose rates. Likewise, you take a “dose” of 1,000 aspirins if you spread that dose over 20 years, but you’re killed if you take the same dose all at once. The dose criterion implicitly assumes no repair, so it is clearly wrong.
Muller, who got the Nobel prize for discovering that X-rays mutate fruit flies, argued in May 1957 to the U.S. Congressional hearings on The Nature of Radioactive Fallout and Its Effects on Man that there is no significant dose rate effect or threshold dose using his fruit fly data, plus some maize plant data on genetic effects of radiation from geneticists. However, short-lived fruit flies and seasonal crops don’t have the DNA repair enzymes like P53, which were only discovered about 20 years later!
The DNA double helix (two strands of DNA facing each other in a spiral) in every cell nucleus in the human body suffers 200,000 single strand breaks and 15 double strand breaks every day. What’s interesting is only 0.007% of natural breaks are double-strand breaks, while 4% of radiation-induced breaks are double strand breaks. This debunks the groupthink myth that DNA damage is due to natural background radiation. It isn’t! If it were, the ratio of single to double strand breaks would be the same for both natural DNA damage, and radiation-induced DNA damage.
It turns out that the natural damage to DNA is mostly due to thermal instability, i.e. 37 °C body temperature, the mechanism being Brownian motion kinetic energy effects, i.e. water molecule bombardment of DNA molecules, related natural free radicals, etc. The cells have DNA repair proteins to rejoin the broken ends of DNA molecules. Single strand breaks don’t cause much risk, because the double helix as a whole remains unbroken. The one broken strand is easily rejoined by a DNA repair enzyme like P53, and all is well.
The cancer risk occurs with double strand breaks, because then the entire double helix is broken off at that point. If you get two double strand breaks occurring quickly, before a DNA repair protein has time to rejoin correctly them, at a very high radiation dose rate, then the loose broken-free segment of DNA might move, reverse, or be lost, and the wrong ends can be joined by accident (like trying to repair a vase after it is smashed up into lots of similar pieces all at once), causing a mutation that can lead to cancer in some cases.
FURTHER READING: SELECTED USEFUL POST REFERENCES IN MY OTHER BLOG
Hiroshima and Nagasaki propaganda debunked by factual evidence which politicians cover up
Civil defense facts from Hiroshima and Nagasaki which the politicians suppress with secrecy laws
There is no significant carbon emission: 97% of the annual release of carbon dioxide is from non-human animals and sealife, according to the 1997 IPCC report, see page 15 of http://vixra.org/pdf/1211.0156v1.pdf Each year, 771 gigatons of carbon is emitted by animals and sealife, compared to only 29 gigatons from human activities. From 1948-2009, NOAA data show a 1% decrease in measured atmospheric humidity (for the full depth of the atmosphere), which is equivalent to a 26% fall in CO2 (because water vapour has 26 times more influence as a greenhouse gas than CO2 does). Therefore, the 25% increase in CO2 during that period has been cancelled out by the fall in water vapour during that same period.
Think of a greenhouse with CO2 injected into it. It gets hotter. Now imagine one with 71% area covered by water, and a roof over 15,000 feet high that can contain cloud cover! The water now evaporates when it warms slightly due to the CO2, and clouds form as a result when the humid air absorbs infrared radiation from the sun and rises bouyantly into it meets cool air high up. The cirrus clouds formed then cut off (attenuate) the infrared radiation, which no longer reaches the low altitude where most of the CO2 lies. So the cloud cover is an automatic self-regulating thermostat that kicks in with “negative feedback” to cancel the effects of injecting CO2.
Earth’s 71% ocean area climate is in effect already saturated with water vapour, which is 26 times more powerful as greenhouse gas as CO2. Injecting CO2 provably (see Dr Roy Spencer’s peer-reviewed but IPCC-censored papers on “negative feedback” from water opposing CO2) precipitates water vapour into cirrus cloud droplets, which increases cloud cover, shadowing and cooling the earth below.
We have a detailed study of the malicious, ignorant, ideologues whose religion is the half-assed egocentric and narcissistic attempt to promote themselves as heroes for “saving” an increasing population of polar bears which eat seals and people. Note that James Enstrom and and Geoffrey Kabat analysed three decades of American Cancer Society data from 1959-89, tracking passive smoking by 118,000 Californians. Second hand “passive smoking” even prolonged in smoke filled homes day after day, caused no significant risk of cancer. The American Cancer Society and Tobacco Related Disease Research Program simply ended their funding. Similar things happened to researchers who proved that radiation is good for you and reduces cancer at low dose rates: the deluded fanatics of communism masquerading as the “politically correct” then simply stopped their funding because they want to stop nuclear power from bettering the lives of billions of human beings.













3 thoughts on “Incidents at Japan’s Fukushima Dai-ichi nuclear reactor Units 1, 2, and 3, and fire at Unit 4, after the 11 March Sendai earthquake and tsunami”