Abstract
α-amylase and α-glucosidase are key enzymes implicated in carbohydrate digestion and their inhibition has been suggested as a powerful approach for regulating blood glucose levels. The present work describes for the first time their inhibition by a group of twelve hydroxylated 2-styrylchromones (2-SC). Our findings revealed that 2-SC display strong systematic inhibition of α-glucosidase rather than α-amylase activity. The number and position of the hydroxy groups in the chromone moiety further modulate the inhibitory profile of the studied compounds, and the derivatives bearing one catechol unit are efficient inhibitors of both enzymes. Enzyme kinetic studies indicate that all active compounds act as competitive inhibitors of α-amylase while most of them behave as non-competitive inhibitors of α-glucosidase. The results are promising and pave the way to further deciphering the potential of this class of compounds as a suitable alternative for the management of type 2 diabetes and its complications.

Similar content being viewed by others
Introduction
According to the International Diabetes Federation (IDF) Atlas records, people with Diabetes mellitus (DM) increased from 436 million (20–79 years old) in 2019 to 537 million in 2021 and this number is predicted to rise to 643 million by 2030 and outnumber 783 million by 2045. Regretfully, besides the long-term debilitating sequels, diabetes is among the top causes of premature death, being responsible for 6.7 million deaths around the world in 2021 [1].
According to the aetiology, DM subdivides into type 1, type 2, gestational and others. Type 2 DM, also known as insulin-resistant or adult-onset diabetes, is the commonest in middle-aged and older people. It occurs when the body does not produce enough insulin or the body’s cells do not respond normally to the insulin. If left untreated, this disorder may cause severe long-term micro- and macrovascular complications that can be life-threatening, such as cardiovascular diseases, peripheral neuropathy, diabetic nephropathy, and retinopathy, among others [2,3,4,5,6].
Commonly used drugs to treat type 2 DM include sulfonylureas, thiazolidinediones, biguanides, meglitinides, glucagon-like peptide-1 agonists, dipeptide peptidase 4 inhibitors, and sodium-glucose cotransporter-2 inhibitors. Another common approach considers lowering the amount of assimilated glucose through supplemented use of inhibitors of key enzymes involved in carbohydrate digestion, such as α-amylase (EC 3.2.1.1) and α-glucosidase (EC 3.2.1.20) [2, 7,8,9,10]. Digestion starts in the mouth, with the activity of salivary α-amylase, promoting the hydrolysis of α-(1,4)-glycosidic bonds of dietary carbohydrates (e.g., starch) to a series of branched glucans and small linear glucans. When the mixture is produced, the chyme reaches the stomach and salivary α-amylase activity ceases because of the acidic environment. Hydrolysis is resumed in the upper part of the small intestine by the release of pancreatic α-amylase. α-Glucosidase at the brush border of the small intestine ultimately catalyzes the hydrolysis of α-(1,4)-glycoside bonds at the non-reductive end and in a smaller extension of α-(1,6) bonds of oligosaccharides, to free glucose and other monosaccharides. In this sense, targeting the action of α-amylase and α-glucosidase enzymes can prevent the excessive production of glucose and be a first-line approach to attain normoglycemia in type 2 DM [11].
Clinically approved inhibitors of α-glucosidase are limited and include acarbose, which also inhibits α-amylase as well as activities of enzymes such as glucoamylase, maltase, sucrase and isomaltase [12,13,14]. Acarbose is prescribed very often but requires co-administration of carbohydrates to exert its effect and reduce the risk of hypoglycemia. In turn, this therapeutic option is associated to undesired side effects at the gastrointestinal tract, as result of the accumulation of undigested carbohydrates in the large intestine, that includes abdominal distension, flatulence and diarrhea [15,16,17,18]. Thus, several efforts have been geared toward developing new and effective alternatives ranging from different classes of natural products to diverse synthetic analogues [12, 19,20,21,22].
Chromones represent a class of disseminated oxygenated heterocyclic compounds with several biological and pharmacological properties, that cover the antidiabetic action [23,24,25], as evidenced by some 2-arylchromones [21, 22]. The small group of chromones known as 2-styrylchromones (2-SC), albeit scarcer in nature, has been largely studied over the past three decades. It includes the isolation of a few natural derivatives, the synthesis of a vast variety of synthetic analogues and the assessment of biological activities such as antibacterial, antifungal, anti-inflammatory, antioxidant, antiviral, and antitumoral, among others [26,27,28]. Nonetheless, to the best of our knowledge, studies on the antidiabetic potential of 2-SC derivatives has not been conducted to date. Thus, we envisage that like 2-arylchromones, the inhibition of the carbohydrate-hydrolyzing enzymes by this class of compounds might be the starting point in the discovery of novel and efficient antidiabetic agents.
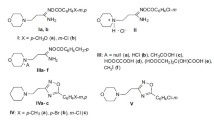
In this sense, the in vitro inhibitory activity, and the type of inhibition against pancreatic α-amylase and yeast α-glucosidase enzymes by a panel of twelve synthetic 2-SC 1-3A-D (Fig. 1), bearing hydroxy groups in different positions of the chromone scaffold, is herein described for the first time.
Results
In vitro α-amylase inhibitory activity
The inhibitory effects of 2-SC 1-3 upon α-amylase were evaluated and compared with the standard inhibitor, acarbose (IC50 = 0.62 ± 0.07 μM). The results varied either according to the substitution in the B-ring of the styryl group or secondly on the substitution pattern at the A-ring of chromone. Those trends allowed us to group them into three sets, as summarized in Table 1. All 2-SC of the first set exhibited inhibitory effects on pancreatic α-amylase activity in a concentration-dependent manner, with IC50 varying from 25.9 to 88 μM. Namely 2-SC 1C (with 5-OH in A-ring) and 1A [with 5,7-(OH)2 in A-ring] were the most effective compounds of the group, with almost similar effects (IC50s of 25.9 ± 0.9 μM and 29 ± 2 μM, respectively) (Table 1 and Fig. 2). Compound 1B (with 7-OH in A-ring) was less efficient and provided an IC50 value of 68 ± 3 μM. Derivative 1D (without substitution in A-ring) was the less active of the group, with an IC50 value of 88 ± 2 μM.
Only 2-SC 2A and 2B from the second set (with 4’-OH in B-ring), have shown a concentration-dependent activity, although the 2-SC 2A (IC50 = 62 ± 3 μM) (Table 1 and Fig. 2) was noticeably more potent than the 2-SC 2B (IC50 = 174 ± 6 μM). Compound 2C presented no ability to inhibit α-amylase up to the highest tested concentration of 200 μM and 2-SC 2D reached only a 33% inhibitory effect for 200 μM concentration.
Set 3 (no substitution in B-ring), comprehended the less efficient group of compounds tested, recording low inhibitory activities from 24% to 48%, up to the maximum tested concentrations (200 μM).
Nevertheless, all active 2-SC showed moderate inhibition against α-amylase activity when compared with the positive control, acarbose (Table 1).
The type of inhibition of the most active derivatives of set 1 (1A-D), set 2 (2A, 2B) and the positive control was deducted from the statistical evaluations of the experimental data fitting to the corresponding Michaelis–Menten kinetics model. Further comparison of experimental data distances to the Lineweaver–Burk double reciprocal plots generated by each fitted model was performed by visual inspection. Compounds 1A-D, 2A and 2B behaved as competitive inhibitors of α-amylase with corresponding kinetic parameters (Vmax, Km, Kic), as shown in Table 2. As an example, Fig. 3 depicts how the Lineweaver–Burk plots generated from parameters in Table 2 superimpose experimental data got for α-amylase activity in the presence of the selected compounds 1D and 2B (see SI for the other Lineweaver–Burk plots). Thus, increasing the inhibitor concentration, the plots present constant Vmax values and increasing values of Km, which is characteristic of competitive inhibitors. As already reported in other studies, a mixed type of inhibition was also recorded for acarbose, where the values of the kinetic parameters Vmax and Km decreased with increasing concentrations of acarbose [11, 21].
Lineweaver–Burk plots of α-amylase inhibition by 2-SC 1D and 2-SC 2B. Marquees correspond to the mean values of the experimental results. Lines were plotted from the fitted model competitive inhibition when (left) Vmax = 53 Δabs/min; Km = 1188 μM; Kic = 70 μM, and (right) Vmax = 51.8 Δabs/min; Km = 1167 μM; Kic = 128 μM
The analysis by ANOVA one-factor of the experimental data showed a precision ranging from 0.9 and 2.4 Δabsorbance (ΔAbs) per min for the tested 2-SC and 1.6 ΔAbs per min for acarbose, as calculated from the within-groups mean square. The same statistical test also recorded for the tested compounds F values of 23-221, higher than the F critical values (ranging from 1.98 to 2.07), corresponding to a p < 0.05 (one-tail probability), as a consequence of its significant effect on the enzymatic activity.
In all cases, the inhibition kinetic model was the best fitted, thus providing the lowest sum of squared residuals after iterative nonlinear regression using the Solver™ supplement.
In vitro α-glucosidase inhibitory activity
The inhibitory activity upon yeast α-glucosidase by 2-SC 1-3 and the positive control, acarbose (IC50 = 528 ± 9 μM), is resumed in Table 1.
All 2-SC derivatives of set 1 proved to inhibit α-glucosidase in a concentration-dependent manner. Derivative 1A was the most effective, presenting an IC50 value of 19 ± 3 μM, being considerably more efficient than the rest of the compounds tested (Table 1 and Fig. 4). Compound 1C was 2.5-fold less active and compound 1B was 4.5-fold less active than 1A, with corresponding IC50 values of 47 ± 2 μM and 86 ± 7 μM, respectively. 2-SC 1D was the less effective α-glucosidase inhibitor of the group, providing an IC50 value of 149 ± 12 μM (Table 1 and Fig. 4).
Unlike to above-referred for α-amylase, all derivatives of set 2 were able to inhibit α-glucosidase in a concentration-dependent manner. Furthermore, 2-SC 2A (IC50 = 29 ± 3 μM) was the most active one and twice more efficient than 2-SC 2B (IC50 = 56 ± 7 μM). Derivative 2C was also able to inhibit α-glucosidase although it was revealed to be less efficient, with an IC50 value of 105 ± 2 μM, and compound 2D was the less active compound of the group, presenting an IC50 value of 136 ± 10 μM (Table 1).
From set 3, only derivatives 3A and 3B exhibited an inhibitory effect dependent on the concentration, with 3A (IC50 = 76 ± 3 μM) being almost twice more active than 3B (IC50 = 143 ± 6 μM). For compounds 3C and 3D it was not possible to achieve the IC50 value, presenting only a slight inhibitory effect of 36 ± 2% and 24 ± 3%, respectively, at the maximum tested concentrations of 200 μM (Table 1).
The IC50 values found for the α-glucosidase inhibitory activity by 2-SC 1-3 vary from 19 up to 149 μM and are significantly lower than the IC50 value found for the positive control, acarbose (IC50 = 528 ± 9 μM) (Table 1).
The inhibition mechanisms of 2-SC 1-3 and the positive control acarbose against α-glucosidase were deduced from the statistical evaluations of the experimental data fitting to the corresponding Michaelis–Menten kinetics model and Lineweaver–Burk plots, using a similar procedure to the already described for α-amylase.
In Table 2 is depicted the results for the type of inhibition against α-glucosidase and the corresponding kinetic constants values (Vmax, Km, Kic and/or Kiu) for the active 2-SC, obtained from nonlinear regression of respective inhibition theoretical models.
The most active derivative, 2-SC 1A, behaved as a competitive inhibitor of α-glucosidase activity and the respective Lineweaver–Burk plot is disclosed in Fig. 5. Thus, it is possible to observe that when the Vmax value remained constant independently of the inhibitor concentration, higher concentrations of the inhibitors led to increasing Km values. Derivative 2B showed a mixed type of inhibition on α-glucosidase activity while the remaining tested compounds exhibited a non-competitive type of inhibition. As an example, the Lineweaver–Burk plot of α-amylase inhibition by the non-competitive inhibitor 2-SC 3A is shown in Fig. 5, where increasing the concentration of compound 3A, Km values remained constant and Vmax values decreased (see SI for the other Lineweaver–Burk plots).
The positive control acarbose exhibited a competitive type of inhibition, once the value of kinetic parameter Km increased and of Vmax remained constant, with increasing concentrations of acarbose (Table 2).
The analysis by ANOVA one-factor of the experimental data showed a precision ranging from 3.7 to 7.4 Δabsorbance (ΔAbs) per min for the tested 2-SC and 2.9 ΔAbs per min for acarbose, as calculated from the within-groups mean square. Moreover, significant effect on the enzymatic activity by all active 2-SC was also recorded since F values of 21-1081 were higher than the F critical values (ranging from 1.85 to 2.02), corresponding to a p < 0.05 (one-tail probability).
Discussion
A wide variety of natural and synthetic compounds have been screened to modulate DM pathways upon inhibition of the carbohydrate hydrolyzing enzymes, α-amylase and α-glucosidase [19, 29,30,31]. Among these, some chromones has been studied regarding inhibition of both enzymes, varying the type and number of substituents attached to their skeleton [19]. As far as known, this is the first report on α-amylase and α-glucosidase inhibitory activity by a range of synthetic 2-SC, which includes twelve derivatives with different substitution patterns (Fig. 1). Thus, compounds are numbered 1-3, in which compounds of set 1 possess a 3′,4′-(OH)2 in B-ring, compounds of set 2 bear a 4′-OH in B-ring and compounds of set 3 presents no substitution in B-ring. The variation in A-ring involves the presence in both 5- and 7-hydroxy groups (derivatives A), 7-OH substitution (derivatives B), 5-OH substitution (derivatives C) and without substitution in A-ring (derivatives D).
Following the obtained results, the inhibitory effects on pancreatic α-amylase activity by 2-SC seem to depend particularly on the OH-substitution pattern at the styryl aromatic ring, B-ring. Compounds of set 1, with a catechol unit [3′,4′-(OH)2] in B-ring, presented a considerably higher effect than those lacking this feature as it can be confirmed by the comparison of the results from set 1 with the those from sets 2 and 3. In set 1 we found the most active compounds of this work, 2-SC 1C, with a 5-hydroxy group of A-ring, presenting an IC50 value of 25.9 ± 0.9 μM and 2-SC 1A, with 5- and 7-hydroxy groups of A-ring, exhibiting an IC50 value of 29 ± 2 μM. Moreover, low efficiency was observed for compounds of set 3 (unsubstituted in B-ring), where it was not possible to achieve the IC50 values of the compounds at the highest tested concentration. Accordingly, it was previously described in in vitro studies that the absence of substituents in B-ring in the several flavonoids was disadvantageous for the inhibitory activity against α-amylase [21, 32, 33].
The presence of the hydroxy substituents in the A-ring also contributes positively to the inhibitory efficiency of 2-SC 1 and 2. Thus, 2-SC 1A (IC50 = 29 ± 2 μM) and 2A (IC50 = 62 ± 3 μM), which possess 5,7-(OH)2 substitution were shown to be more effective inhibitors than 2-SC 1D (IC50 = 88 ± 2 μM) and 2D (33% inhibition at 200 μM), that are unsubstituted in A-ring. A similar conclusion was taken by our group when a panel of structurally-related flavones was tested, lacking the Cα = Cβ double bond of 2-SC. Luteolin [5,7,3′,4′-(OH)4 flavone] (IC50 = 78 ± 3 μM) and apigenin [5,7,4′-(OH)3 flavone] (IC50 = 122 ± 7 μM) were more active than 3′,4′-(OH)2 flavone (46% inhibition at 200 μM) and 4′-OH flavone (no inhibitory effect) [21]. Even more, comparing the results of the above-referred 2-SC with the related flavones we can notice that 2-SC are more active against α-amylase than flavones, leading to infer that the presence of the Cα = Cβ double bond is relevant to enhance the inhibitory effect. This fact points to a likely contribution of the styryl moiety to molecular stabilization, increasing the compound’s inhibitory activity [34].
The most active compounds were 2-SC 1A-C and 2A, with inhibitory order potency as 2-SC 1C (IC50 = 25.9 ± 0.9 μM) ≈ 2-SC 1A (IC50 = 29 ± 2 μM) > 2-SC 2A (IC50 = 62 ± 3 μM) ≈ 2-SC 1B (IC50 = 68 ± 3 μM). Nevertheless, the positive control, acarbose (IC50 = 0.62 ± 0.07 μM), was a noticeably more efficient inhibitor of α-amylase than the tested 2-SC.
The mechanism explaining α-amylase inhibition and the kinetic parameters of all active compounds and the positive control acarbose were further assessed using the generalized Michaelis-Menten model and its simplifications. Both strategies revealed that compounds 1A-D, 2A and 2B act via the competitive type of inhibition i.e., compete directly for the catalytic site of α-amylase. In this circumstance, higher amounts of substrate would be required to generate similar reaction product concentrations in the same period. Acarbose displayed a mixed-type inhibition mechanism, as previously described by other authors for the inhibition of pancreatic α-amylase enzymatic activity [11, 21, 33, 35].
Concerning the hydrolytic catalysis promoted by α-glucosidase and regarding its inhibition by the 2-SC tested, whatever the considered sets 1-3, derivatives A (possessing 5,7-(OH)2 substitution) were the most active compounds. Even so, 2-SC 1A (IC50 = 19 ± 3 μM) was 1.5 times more active than 2-SC 2A (IC50 = 29 ± 3 μM) and 4 times more active than 2-SC 3A (IC50 = 76 ± 3 μM). These results lead us to infer the importance, simultaneously, of the 5-OH and 7-OH groups for the high inhibitory activity towards α-glucosidase. This is consistent with previous findings pinpointing the presence of hydroxy groups in the A-ring as pivotal to the inhibitory profile of flavones. Accordingly, luteolin [5,7,3′,4′-(OH)4 flavone] (IC50 = 46 ± 6 μM) and apigenin [5,7,4′-(OH)3 flavone] (IC50 = 82 ± 6 μM) were more efficient α-glucosidase inhibitors than the corresponding flavones with a single hydroxyl group in A-ring and even more active than those lacking this substitution on A-ring [22].
In contrast, derivatives D (without OH groups in A-ring) were the less active of their groups, as can be observed by comparison of the IC50 values of 2-SC 1D (IC50 = 149 ± 12 μM), 2-SC 2D (IC50 = 136 ± 10 μM) and 2-SC 3D (24% inhibition at 200 μM) with those of the respective derivatives A. A similar conclusion was drawn by us and other groups when studied the inhibitory effects of flavonoids substituted with hydroxy groups [22, 36, 37]. A dramatic loss of the α-glucosidase inhibitory efficiency was verified for flavones missing any hydroxy group on A-ring, as noticed for 3′,4′-(OH)2 flavone (32% inhibition at 200 μM), 4′-OH flavone and parent flavone that were not active for the maximum tested concentration of 200 μM [22].
The presence of a 7-OH group in the A-ring of 2-SC contributed positively to the α-glucosidase inhibitory activity since all derivatives B were able to inhibit this enzyme, with inhibitory order potency as 2-SC 2B (IC50 = 56 ± 7 μM) > 2-SC 1B (IC50 = 86 ± 7 μM) > 2-SC 3B (IC50 = 143 ± 6 μM). The presence of the 5-OH group in the A-ring also influenced the inhibitory effects by 2-SC and was more notorious in derivative 1C (IC50 = 47 ± 2 μM), twice more active than derivative 2C (IC50 = 105 ± 2 μM).
Another important feature can be taken from the comparison of the results from the inhibitory activity of 2-SC and those of the corresponding flavones. Analyzing the IC50 values of the most active 2-SC 1A (IC50 = 19 ± 3 μM), 2A (IC50 = 29 ± 3 μM), 1C (IC50 = 47 ± 2 μM) and 2B (IC50 = 56 ± 7 μM) we can state that were considerably lower than those of the corresponding flavones: luteolin [5,7,3′,4′-(OH)4 flavone] (IC50 = 46 ± 6 μM); apigenin [5,7,4′-(OH)3 flavone] (IC50 = 82 ± 6 μM); 5,3′,4′-(OH)3 flavone (IC50 = 66 ± 7 μM) and 7,4′-(OH)2 flavone (IC50 ≈ 200 μM), clear evidence of the importance of Cα = Cβ double bond for the high inhibitory profile of 2-SC [22, 34].
Unlike the α-amylase inhibitory assay, all active 2-SC were able to inhibit α-glucosidase in a very efficient manner, with corresponding IC50 values ranging from 19 to 149 μM, being the most active 2-SC 1A (IC50 = 19 ± 3 μM), more than 25 times more active than the positive control, acarbose (IC50 = 528 ± 9 μM). A strong inhibition of α-glucosidase combined with a mild inhibitory activity against α-amylase will avoid a prolonged inhibition of starch hydrolysis and the accumulation of undigested carbohydrates in the colon, responsible for the severe gastrointestinal complications registered in type 2 DM patients when using this therapeutic strategy [13, 19].
The type of α-glucosidase inhibition and the kinetic constants of all active 2-SC and of the positive control, acarbose, were also evaluated, using Lineweaver–Burk plots and Solver supplement of Excel Microsoft Office™. The two methods were in accordance and showed that 2-SC 1A behaved as a competitive inhibitor, meaning that this compound competes directly with the substrate for the activity of the α-glucosidase enzyme. The mixed type of inhibition exhibited by derivative 2B is representative that the compound can bind to the free enzyme or to enzyme-substrate complex but has a greater affinity for one state or the other. The remaining tested compounds displayed a non-competitive type of inhibition on α-glucosidase activity, meaning that the compounds can bind with the same affinity to free enzyme and enzyme-substrate complex, resulting in a decrease of the enzymatic activity that cannot be overcome by increasing the substrate concentration.
Concerning the positive control acarbose, a competitive type of inhibition mechanism was observed, which is corroborated by other authors [22, 35].
Conclusions
This study has demonstrated for the first time the in vitro inhibition of the carbohydrate-hydrolyzing enzymes α-amylase and α-glucosidase by a group of twelve 2-SC. Generally, 2-SC displayed lower inhibitory activity than the positive control acarbose against α-amylase, but higher inhibitory effects were recorded against α-glucosidase. It was possible to infer that the number and position of the hydroxy groups largely affected the inhibitory profile of the studied compounds, being derivatives of set 1 efficient inhibitor of both enzymes. In addition, all derivatives of set 2 were active against α-glucosidase while non-activity was verified for the derivatives of set 3 against the α-amylase activity. Furthermore, all active 2-SC showed a competitive type of inhibition for α-amylase while a non-competitive inhibition mechanism was recorded against α-glucosidase for most of the tested 2-SC, except for derivative 1A and 2B, that acted via competitive and mixed type of inhibition, respectively.
In summary, the promising results of 2-SC as α-amylase and α-glucosidase inhibitors and the structure–activity relationships established offers a new challenge in the pursuit of novel leading molecules for targeting these key enzymes enrolled in the management of type 2 DM.
Materials and methods
Materials and reagents
The following reagents were obtained from Sigma-Aldrich Co. LLC (St. Louis, MO): α-amylase from porcine pancreas, α-glucosidase from Saccharomyces cerevisiae, acarbose, 2-chloro-4-nitrophenyl-α-D-maltotrioside (CNPG3), dimethylsulfoxide (DMSO), p-nitrophenyl-α-D-glucopyranoside (pNPG), sodium hydrogen phosphate and sodium dihydrogen phosphate. 2-SC 1-3 (Fig. 1) were synthesized as previously described in the literature [38]. For the enzymatic assays, 2-SC 1-3 were dissolved in DMSO (the final concentration of DMSO in the reaction mixture was <5%). The amount of DMSO used had no interference with the assays. A multimode microplate reader (Synergy HT, BIO-TEK) with temperature control capacity was used to perform the spectrophotometric readings in all the assays.
In vitro α-amylase inhibitory activity assay
The α-amylase inhibition assay by each one of the 2-SC 1-3 (0–200 μM) was performed according to a previously reported method, in which the hydrolysis of the CNPG3 substrate (500 μM) is mediated by the enzyme (0.1 U/mL) into 2-chloro-4-nitrophenol (CNP), 2-chloro-4-nitrophenyl-α-D-maltoside (CNPG2), maltotriose and glucose [21]. Results represent at least three independent experiments and were expressed as mean% inhibition ±standard error of the mean (SEM) (see Eq. 1 below). For the blank assay, both the sample and the enzyme solutions were replaced by equal volumes of the solvent (DMSO and phosphate buffer, respectively). The control corresponded to the assay where the sample was replaced by an equal volume of DMSO. Acarbose (0–5 μM) was used as the positive control.
Inhibitory kinetic analysis of α-amylase
The determination of inhibitory mechanisms was performed for the most active 2-SC, which comprised all derivatives of set 1 and derivatives A and B of set 2. The concentrations used were respectively: 1A (10–25 μM); 1B (25-60 μM), 1C (6.25–25 μM), 1D (50–100 μM), 2A (30–75 μM) and 2B (50–150 μM), and between 0.25 and 1 μM for the positive control, acarbose. The remaining 2-SC showed residual activity in preliminary assays at the highest tested concentration of 200 μM.
The methodoly involves a previous described technique in which the compounds tested 2-SC 1A-D and 2A-B were incubated at 37 °C with the enzyme (0.1 U/mL) and the substrate CNPG3 (in final concentrations of 250, 500 and 1000 μM) and the enzymatic kinetic monitored spectrophotometrically at 405 nm, after 30 min of reaction [21].
The experimental results were fitted by nonlinear least squares regression to the generalized Michaelis-Menten model equation [39]. Iterative optimization of parameter values in each model was performed by Solver™ supplement of Excel Microsoft Office™, as described by Bezerra et al. [40] and Dias et al. [41].
The inhibition mechanism was given through charts of the predicted lines of reciprocal of maximum velocity (1/Vmax) against the reciprocal of 2-SC concentrations (1/[S]). Vmax represents the maximum achievable velocity when 0.1 μ/mL of enzyme is used; Km, Michaelis–Menten constant in mM, Kic = inhibitor dissociation constant of enzyme inhibitor expressed in μM−1, and Kiu = inhibitor dissociation constant of enzyme–substrate–inhibitor complex expressed in μM−1 [42, 43].
In vitro α-glucosidase inhibitory activity assay
The α-glucosidase inhibition assay by each one of the 2-SC 1-3 (0–200 μM) was performed according to the reported method with slight modifications [22]. The assay was carried out by monitoring the α-glucosidase-mediated (0.05 μ/mL) transformation of the substrate pNPG (600 μM) into α-D-glucose and α-nitrophenol, spectrophotometrically at 405 nm, after 30 min of reaction. Both, the raw data and the results enabled were obtained as already described before for α-amylase. Acarbose (0–3000 μM) was used as a positive control.
Inhibitory kinetic analysis of α-glucosidase
The determination of inhibitory mechanisms was performed for all tested 2-SC, except 3C and 3D which showed residual activity at the highest tested concentration of 200 μM. The following concentrations were used: 1A (5–20 μM), 1B (25–75 μM), 1C (20–40 μM), 1D (50–150 μM), 2A (20–60 μM), 2B (25–75 μM), 2C (75–110 μM), 2D (50–100 μM), 3A (50–75 μM) and 3B (100–150 μM), and between 250 and 1500 μM for the positive control, acarbose. Experimentally, the enzyme (0.05 μ/mL) was incubated at 37 °C with the mentioned 2-SC 1-3 and the substrate pNPG (in final concentrations of 300, 600 and 1200 μM). The corresponding increases in absorbance values were followed at the wavelength at 405 nm for 30 min.
The study of the inhibition type of the tested 2-SC 1-3 was performed using the nonlinear regression Michaelis–Menten enzyme kinetics and the corresponding Lineweaver–Burk double reciprocal plots. Solver™ supplement of Excel Microsoft Office™ was used to estimate the kinetic parameters and the prediction of the mechanisms of inhibition, as previously described in Section 5.3 for the α-amylase inhibitory kinetic analysis (Table 2) [22].
Statistical analysis
The results of the in vitro inhibition of the 2-SC 1-3 on pancreatic α-amylase and yeast α-glucosidase catalysis are expressed as mean ± SEM. A statistical comparison between the active chromones was performed using ANOVA. Differences among the groups were compared by Tukey test, with a p ≤ 0.05 considered statistically significant. All the statistical analyses were performed using GraphPad Prism™ (version 5.0; GraphPad Software). The type of inhibition was established by comparison among the models using the Solver™ supplement. The extra sum-of-squares F test [44] and the Akaike information criterion (AIC) test [45] confirmed the inhibition mechanisms. The Jackknife procedure helped to determine the error of the kinetic constants values.
References
International Diabetes Federation. IDF Diabetes Atlas, 90th Edition 2021. Available from: http://www.diabetesatlas.org/ Accessed 10 Sept 2023.
Chatterjee S, Khunti K, Davies MJ. Type 2 diabetes. Lancet. 2017;389:2239–51.
Baynes HW. Classification, pathophysiology, diagnosis and management of diabetes mellitus. J Diabetes Metab 2015;6:541.
Piero MN, Nzaro GM, Njagi JM. Diabetes mellitus – a devastating metabolic disorder. Asian J Biomed Pharm Sci 2014;40:1–7.
American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2014;37:S81–90.
Siddiqui AA, Siddiqui SA, Ahmad S, Siddiqui S, Ahsan I, Sahu K. Diabetes: mechanism, pathophysiology and management - a review. Int J Drug Dev Res 2013;5:1–23.
Duarte AM, Guarino MP, Barroso S, Gil MM. Phytopharmacological strategies in the management of type 2 diabetes. Mellit Foods. 2020;9:271.
Chikara G, Sharma PK, Dwivedi P, Charan J, Ambwani S, Singh S. A narrative review of potential future antidiabetic drugs: should we expect more? Indian J Clin Biochem 2018;33:121–31.
Saeedi M, Hadjiakhondi A, Nabavi SM, Manayi A. Heterocyclic compounds: effective α-amylase and α-glucosidase inhibitors. Curr Top Med Chem 2017;17:428–440.
Chaudhury A, Duvoor C, Dendi VSR, Kraleti S, Chada A, Ravilla R, Marco A, Shekhawat NS, Montales MT, Kuriakose K, Sasapu A, Beebe A, Patil N, Musham CK, Lohani GP, Mirza W. Clinical review of antidiabetic drugs: implications for type 2 diabetes mellitus management. Front Endocrinol 2017;8:12.
Santos CMM, Proença C, Freitas M, Araújo AN, Silva AMS, Fernandes E. Inhibition of the carbohydrate-hydrolyzing enzymes α-amylase and α-glucosidase by hydroxylated xanthones. Food Funct 2022;13:7930–41.
Dhameja M, Gupta P. Synthetic heterocyclic candidates as promising α-glucosidase inhibitors: an overview. Eur J Med Chem 2019;176:343–77.
Santos CMM, Freitas M, Fernandes E. A comprehensive review on xanthone derivatives as α-glucosidase inhibitors. Eur J Med Chem 2018;157:1460–79.
Ghani U. Re-exploring promising α-glucosidase inhibitors for potential development into oral anti-diabetic drugs: finding needle in the haystack. Eur J Med Chem 2015;103:133–62.
Drugbank, Available from: https://go.drugbank.com Accessed 10 Sep 2023.
Kokil GR, Rewatkar PV, Verma A, Thareja S, Naik SR. Pharmacology and chemistry of diabetes mellitus and antidiabetic drugs: a critical review. Curr Med Chem 2010;17:4405–23.
Hossain MA, Pervin R. In Current antidiabetic drugs: review of their efficacy and safety, nutritional and therapeutic interventions for diabetes and metabolic syndrome, Chapter 34, 2nd Ed., 2018, pp. 455–73.
Asano N. Sugar-mimicking glycosides inhibitors: bioactivity and application: a review. Cell Mol Life Sci 2009;66:1479–92.
Proença C, Ribeiro D, Freitas M, Fernandes E. Flavonoids as potential agents in the management of type 2 diabetes through the modulation of α-amylase and α-glucosidase activity, a review. Crit Rev Food Sci Nutr 2022;62:3137–207.
Dirir AM, Daou M, Yousef AF, Yousef LF. A review of alpha-glucosidase inhibitors from plants as potential candidates for the treatment of type-2 diabetes. Phytochem Rev 2022;21:1049–79.
Proença C, Freitas M, Ribeiro D, Tomé SM, Oliveira EFT, Viegas MF, Araújo AN, Ramos MJ, Silva AMS, Fernandes PA, Fernandes E. Evaluation of a flavonoids library for inhibition of pancreatic α-amylase towards a structure–activity relationship. J Enz Inhib Med Chem 2019;34:577–88.
Proença C, Freitas M, Ribeiro D, Oliveira EFT, Sousa JLC, Tomé SM, Ramos MJ, Silva AMS, Fernandes PA, Fernandes E. α-Glucosidase inhibition by flavonoids: an in vitro and in silico structure–activity relationship study. J Enz Inhib Med Chem 2017;32:1216–28.
Benny AT, Arikkatt SD, Vazhappilly CG, Kannadasan S, Thomas R, Leelabaiamma MSN, Radhakrishnan EK, Shanmugam P. Chromone, a privileged scaffold in drug discovery: developments in the synthesis and bioactivity. Mini Rev Med Chem 2022;22:1030–63.
Gaspar A, Matos MJ, Garrido J, Uriarte E, Borges F. Chromone: a valid scaffold in medicinal chemistry. Chem Rev 2014;114:4960–92.
Keri RS, Budagumpi S, Pai RK, Balakrishna RG. Chromones as a privileged scaffold in drug discovery: a review. Eur J Med Chem 2014;78:340–74.
Lucas M, Freitas M, Silva AMS, Fernandes E, Ribeiro D. Styrylchromones: biological activities and structure-activity relationship. Oxid Med Cell Longev 2021;2804521:47.
Santos CMM, Silva AMS. An overview of 2-styrylchromones: natural occurrence, synthesis, reactivity and biological properties. Eur J Org Chem. 2017;2017:3115–33.
Gomes A, Freitas M, Fernandes E, Lima JLFC. Biological activities of 2-styrylchromones. Mini-Rev Med Chem 2010;10:1–7.
Kaur N, Kumar V, Nayak SK, Wadhwa P, Kaur P, Sahu SK. Alpha-amylase as molecular target for treatment of diabetes mellitus: a comprehensive review. Chem Biol Drug Des 2021;98:539–60.
Bashary R, Vyas M, Nayak SK, Suttee A, Verma S, Narang R, Khatik GL. An insight of alpha-amylase inhibitors as a valuable tool in the management of type 2 diabetes mellitus. Curr Diabetes Rev 2020;16:117–36.
Teng H, Chen L. α-Glucosidase and α-amylase inhibitors from seed oil: a review of liposoluble substance to treat diabetes. Crit Rev Food Sci Nutr 2017;57:3438–48.
Cao H, Chen X. Structures required of flavonoids for inhibiting digestive enzymes. Anticancer Agents Med Chem 2012;12:929–39.
Rocha S, Sousa A, Ribeiro S, Correia CM, Silva VLM, Santos CMM, Silva AMS, Araújo AN, Fernandes E, Freitas M. A study towards drug discovery for the management of type 2 diabetes mellitus through inhibition of the carbohydrate-hydrolyzing enzymes α-amylase and α-glucosidase by chalcone derivatives. Food Funct 2019;10:5510–20.
Bhurta D, Bharate SB. Styryl group, a friend or foe in medicinal chemistry. ChemMedChem. 2022;17:e202100706.
Kim MJ, Lee SB, Lee HS, Lee SY, Baek JS, Kim D, Moon TW, Robyt JF, Park K-H. Comparative study of the inhibition of α-glucosidase, α-amylase, and cyclomaltodextrin glucanosyltransferase by acarbose, isoacarbose, and acarviosine-glucose. Arch Biochem Biophys 1999;371:277–83.
Gao H, Nishioka T, Kawabata J, Kasai T. Structure-activity relationships for alpha-glucosidase inhibition of baicalein, 5,6,7-trihydroxyflavone: the effect of A-ring substitution. Biosci Biotechnol Biochem 2004;68:369–75.
Gu C, Zhang H, Putri CY, Ng K. Evaluation of α-amylase and α-glucosidase inhibitory activity of flavonoids. Int J Food Nutr Sci 2015;2:1–6.
Santos CMM, Silva AMS, Cavaleiro JAS. Synthesis of new hydroxy-2-styrylchromones. Eur J Org Chem. 2003;2003:4575–85.
Rocha S, Lucas M, Silva VLM, Gomes PMO, Silva AMS, Araújo AN, Aniceto N, Guedes RC, Corvo ML, Fernandes E, Freitas M. Pyrazoles as novel protein tyrosine phosphatase 1B (PTP1B) inhibitors: an in vitro and in silico study. Int J Biol Macromol 2021;181:1171–82.
Bezerra RMF, Fraga I, Dias AA. Utilization of integrated Michaelis-Menten equations for enzyme inhibition diagnosis and determination of kinetic constants using Solver supplement of Microsoft Office Excel. Comput Methods Prog Biomed 2013;109:26–31.
Dias AA, Pinto PA, Fraga I, Bezerra RMF. Diagnosis of enzyme inhibition using excel solver: a combined dry and wet laboratory exercise. J Chem Educ 2014;91:1017–21.
Banerjee S. Inhibition of mackerel (Scomber scombrus) muscle lipoxygenase by green tea polyphenols. Food Res Int 2006;39:486–91.
Nelson, DL, Cox, MM. Enzyme kinetics as an approach to understanding mechanism. In Lehninger principles of biochemistry, 6th Ed, New York: W. H. Freeman & Co., 2014, pp. 200–12.
Burguillo FJ, Wright AJ, Bardsley WG. Use of the F test for determining the degree of enzyme-kinetic and ligand-binding data. A Monte Carlo simulation study. Biochem J. 1983;211:23–34.
Yamaoka K, Nakagawa T, Uno T. Application of Akaike’s information criterion (AIC) in the evaluation of linear pharmacokinetic equations. J Pharmacokinet Biopharm 1978;6:165–175.
Acknowledgements
This work received support from PT national funds (FCT/MCTES, Fundação para a Ciência e Tecnologia and Ministério da Ciência, Tecnologia e Ensino Superior) through the projects of CIMO (UIDB/00690/2020 and), SusTEC (LA/P/0007/2021) and REQUIMTE (UIDB/50006/2020 and UIDP/50006/2020) and also through the project EXPL/MED-QUI/0815/2021. MF acknowledges her CEEC Individual 2020.04126.CEECIND/CP1596/CT0006 and LAQV-REQUIMTE for her contract under the reference LA/P/0008/2020. CP acknowledges funding from FCT and MCTES through national funds and COMPETE, grant number PTDC/MED-QUI/29243/2017-POCI-01-0145-FEDER-029243.
Author contributions
Conceptualization, C.M.M.S., A.M.S.S. and E.F.; writing-original draft preparation, C.M.M.S., M.F.; writing-review and editing, C.M.M.S., C.P., M.F., A.N.A. A.M.S.S. and E.F.; supervision, M.F. and E.F. All authors have read and agreed to the published version of the manuscript.
Funding
This work received support from national funds (FCT/MCTES, Fundação para a Ciência e Tecnologia and Ministério da Ciência, Tecnologia e Ensino Superior) through the projects of CIMO (UIDB/00690/2020 and), SusTEC (LA/P/0007/2021) and REQUIMTE (UIDB/50006/2020 and UIDP/50006/2020). This work also received financial support from the project EXPL/MED-QUI/0815/2021, with funding from FCT/MCTES through national funds, and “Programa Operacional Competitividade e Internacionalização” (COMPETE). Open access funding provided by FCT|FCCN (b-on).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Santos, C.M.M., Proença, C., Freitas, M. et al. 2-Styrylchromones as inhibitors of α-amylase and α-glucosidase enzymes for the management of type 2 diabetes mellitus. Med Chem Res 33, 600–610 (2024). https://doi.org/10.1007/s00044-024-03200-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-024-03200-8