Animal Behavior: Whale breaching says it loud and clear
From marine biologists on their first field trip to the most jaded sailors, witnessing whales leaping above the sea surface – a behavior called breaching – rarely fails to elicit child-like wonder (Würsig and Whitehead, 2018). But why do whales breach? Is it, for instance, to dislodge external parasites, or could it simply be for play? Since these cetaceans often jump near each other, scientists have long thought that breaching might be a form of communication, a way for a whale to say: “Look how strong I am, I can propel my huge body right out of the water!” Beyond physical strength, this would also convey information about the animal’s genetic fitness – in other words: “You really should consider me as a potential mate!”
Gazelles that conspicuously leap directly in front of predators, peacocks which sport ridiculously huge tails, and even humans who smoke cigarettes or cover their bodies with tattoos are all prime examples of so-called ‘handicap signals’. These traits or behaviors are energy consuming or risky, but they demonstrate that the individual can bear these costs and still thrive (Zahavi, 1977). For a signal to be ‘honest’, however, it must entail a genuine expense to the animal. Until recently, it was not clear how much energy a breach would truly require from a whale, and how this was limited by the length of the animal, its swimming speed and other factors (Figure 1).
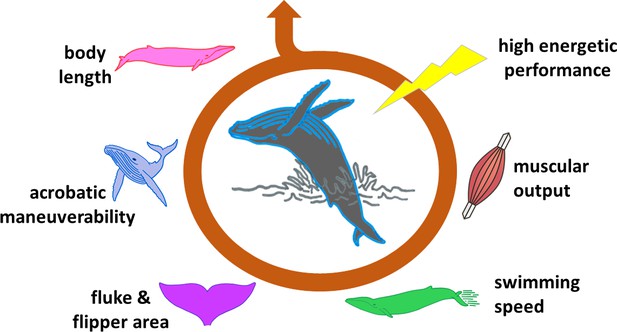
The ability of a whale to breach is influenced by many factors.
Breaching is an energetically expensive behavior which depends on a number of interconnected elements. The length of the animal is one of the most limiting factors, affecting, for instance, body maneuverability. Shorter whales require lower swimming speeds to breach, and therefore lower muscle power. The surface area of flukes and flippers also influences how fast a whale can swim and turn. These and other features determine whether the animal has the capacity to expend a large amount of energy in a very short time (high energetic performance), and is therefore able to breach.
Now, in eLife, Paolo Segre from Stanford University and colleagues based in Italy, Portugal, the United States and Denmark report the results of a study on the energetic limits of breaching (Segre et al., 2020). The 18-member team used long poles and suction cups to temporarily attach sensors onto the bodies of five species of whales. The instruments recorded 187 breaches, tracking water depth and temperature as well as capturing the position of the whales over time and video clips before and during the jumps (Goldbogen et al., 2017; Cade et al., 2018).
Using these data to reconstruct the trajectory of breaching whales showed, for example, that young humpback whales often roll on their sides as they exit the water, while the adults usually emerge right-side up or upside-down. Swimming speeds reached a brisk 8–9 meters per second, but the large amounts of energy required for each breach did not stop some of the whales from leaping repeatedly: indeed, one juvenile humpback breached 52 times in just over four hours.
To calculate how much energy a whale spends on a breach, Segre et al. considered a series of factors that included metabolic rates, the energy needed for muscular contractions (Fish, 1998), the frequency of the tail stroke, and variables that influence water resistance (such as viscosity). Calculations showed that the energy costs of breaching depended on muscle power, which in turn is related to the length of the whale (Kahane-Rapport and Goldbogen, 2018; Gough et al., 2019). Large specimens must swim faster to clear the surface, requiring their muscles to generate tremendous power: a 15-meter-long humpback whale needs nearly ten times more energy per breach than a whale that is 8 meters long. In fact, in the few seconds that it takes to breach, the 15-meter whale can expend as much energy as a 60-kilogram human would during a marathon. Segre et al. therefore contend that a breach may represent the “most expensive burst maneuver” in all of nature, pushing the boundaries of muscular performance and providing an honest signal of a whale’s general health.
Overall, it is the length of a whale (rather than its mass) that appears to be the biggest obstacle to breaching: this may explain differences in breaching behavior between species of similar mass, but distinct builds. Blue whales, the longest species, are limited by how much power their muscles can deliver in short bursts and by the hydrodynamics of their tails. On the other hand, right whales, another large species, have short and chunky bodies, wider tails, and a thick, buoyant fat layer, all of which could help with breaching. In the end, species like humpbacks – famous for their agility as much as their complex social structure (Segre et al., 2019) – might be best suited to breaching. Regardless, there is no doubt that breaching comes at great expense for all types of whales: so next time you marvel at a leaping cetacean, consider the breach’s dazzling metabolic cost as well as its eye-widening beauty.
References
-
Determining forward speed from accelerometer jiggle in aquatic environmentsThe Journal of Experimental Biology 221:jeb170449.https://doi.org/10.1242/jeb.170449
-
Comparative kinematics and hydrodynamics of odontocete cetaceans: morphological and ecological correlates with swimming performanceThe Journal of Experimental Biology 201:2867–2877.
-
Scaling of swimming performance in baleen whalesThe Journal of Experimental Biology 222:jeb204172.https://doi.org/10.1242/jeb.204172
-
Allometric scaling of morphology and engulfment capacity in rorqual whalesJournal of Morphology 279:1256–1268.https://doi.org/10.1002/jmor.20846
-
Body flexibility enhances maneuverability in the world's largest predatorIntegrative and Comparative Biology 59:48–60.https://doi.org/10.1093/icb/icy121
-
Encyclopedia of Marine Mammals6–10, Aerial behavior, Encyclopedia of Marine Mammals, San Diego, Academic Press, 10.1016/b978-0-12-804327-1.00040-6.
-
The cost of honesty (further remarks on the handicap principle)Journal of Theoretical Biology 67:603–605.https://doi.org/10.1016/0022-5193(77)90061-3
Article and author information
Author details
Publication history
- Version of Record published: March 11, 2020 (version 1)
Copyright
© 2020, Werth and Lemon
This article is distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use and redistribution provided that the original author and source are credited.
Metrics
-
- 2,225
- views
-
- 125
- downloads
-
- 1
- citations
Views, downloads and citations are aggregated across all versions of this paper published by eLife.
Download links
Downloads (link to download the article as PDF)
Open citations (links to open the citations from this article in various online reference manager services)
Cite this article (links to download the citations from this article in formats compatible with various reference manager tools)
Further reading
-
- Computational and Systems Biology
- Physics of Living Systems
Locomotion is a fundamental behavior of Caenorhabditis elegans (C. elegans). Previous works on kinetic simulations of animals helped researchers understand the physical mechanisms of locomotion and the muscle-controlling principles of neuronal circuits as an actuator part. It has yet to be understood how C. elegans utilizes the frictional forces caused by the tension of its muscles to perform sequenced locomotive behaviors. Here, we present a two-dimensional rigid body chain model for the locomotion of C. elegans by developing Newtonian equations of motion for each body segment of C. elegans. Having accounted for friction-coefficients of the surrounding environment, elastic constants of C. elegans, and its kymogram from experiments, our kinetic model (ElegansBot) reproduced various locomotion of C. elegans such as, but not limited to, forward-backward-(omega turn)-forward locomotion constituting escaping behavior and delta-turn navigation. Additionally, ElegansBot precisely quantified the forces acting on each body segment of C. elegans to allow investigation of the force distribution. This model will facilitate our understanding of the detailed mechanism of various locomotive behaviors at any given friction-coefficients of the surrounding environment. Furthermore, as the model ensures the performance of realistic behavior, it can be used to research actuator-controller interaction between muscles and neuronal circuits.
-
- Physics of Living Systems
Termites build complex nests which are an impressive example of self-organization. We know that the coordinated actions involved in the construction of these nests by multiple individuals are primarily mediated by signals and cues embedded in the structure of the nest itself. However, to date there is still no scientific consensus about the nature of the stimuli that guide termite construction, and how they are sensed by termites. In order to address these questions, we studied the early building behavior of Coptotermes gestroi termites in artificial arenas, decorated with topographic cues to stimulate construction. Pellet collections were evenly distributed across the experimental setup, compatible with a collection mechanism that is not affected by local topography, but only by the distribution of termite occupancy (termites pick pellets at the positions where they are). Conversely, pellet depositions were concentrated at locations of high surface curvature and at the boundaries between different types of substrate. The single feature shared by all pellet deposition regions was that they correspond to local maxima in the evaporation flux. We can show analytically and we confirm experimentally that evaporation flux is directly proportional to the local curvature of nest surfaces. Taken together, our results indicate that surface curvature is sufficient to organize termite building activity and that termites likely sense curvature indirectly through substrate evaporation. Our findings reconcile the apparently discordant results of previous studies.






